Laksenæringen bruker i dag en rekke strategier for å bekjempe lakselus, men avlusingsmetoder har utfordringer på grunn av dårlig effekt, dårlig velferd og miljøpåvirkninger, eller potensiell utvikling av resistens mot medisinske behandlinger. De tre avlusingsmetodene som oftest brukes (termisk, mekanisk og ferskvann), brukt alene eller i kombinasjon, har ikke blitt systematisk studert eller sammenlignet slik at bruken av er optimaliseres. Dette prosjektet har samlet eksperimentelle data på effekt av avlusing og velferdseffekter med bruk av enkelt- og kombinasjonsbehandlinger ved å bruke disse tre metodene i karforsøk. Enkeltbehandlinger med ferskvann eller ferskvann i kombinasjon med andre behandlinger hadde størst avlusingseffekt. Fiskens velferd ble redusert i alle behandlingsgruppene gjennom forsøkene, og statistisk analyse viste ingen konsistent forskjell mellom prosedyrekontroller og individuelle eller kombinerte behandlinger. Funnene tyder på at enhver behandling som brukes alene eller i kombinasjon har en negativ innvirkning på fiskevelferden og at det er håndteringen snarere enn behandlingen som er den største bidragsyteren til dårlige resultater. Imidlertid resulterte behandlingene med et 34 °C termisk bad i de dårligste velferdsresultatene, de laveste konditionsfaktorene og laveste veksthastigheter, noe som tyder på at 34 °C termiskbadet er spesielt skadelig for fiskevelferden. Prosjektet undersøkte også velferdseffekten av avlusing på kommersielle lokaliteter ved å systematisere data fra en produksjonssyklus ved flere anlegg. Mens avlusingsstrategiene var mer avhengig av produksjonsmål og regulatoriske bestemmelser, viste dataene også at dødeligheten øker etter alle håndteringshendelser og avlusingsoperasjoner. Til slutt ble toleransenivåene for temperatur og lav saltholdighet, og evnen for disse toleransenivåene til å endre seg ved eksponering, undersøkt hos lakseluslarver. Tre stammer ble undersøkt, og mens deres temperaturtoleransenivåer var like, ble det funnet signifikante forskjeller i saltholdighetstoleransenivåer mellom stammer. En mer detaljert undersøkelse på epigenetisk nivå (spesifikt rettet mot 5-mC DNA-epimetylering) avslørte signifikante forskjeller mellom grupper, og indikerte potensialet for å finne saltholdighetstoleransemarkører i eldre lusestadier. Dette prosjektet ga komparative data om avlusingsmetoder som øker vår kunnskap om avlusings- og velferdseffekt på enkelt- og kombinasjonsmetoder, med ulike faktorer tatt i betraktning, som bør verifiseres ytterligere med prosedyrer i kommersiell skala.
Optimising delousing strategies: developing best practice recommendations for maximal efficacy and positive welfare
— Final Report
Report series:
Rapport fra havforskningen 2023-29
ISSN: 1893-4536
Published: 29.06.2023
Project No.: 15769
On request by: FHF
Reference: 901687
Research group(s):
Dyrevelferd
Subject:
Fiskevelferd
Program:
Fremtidens havbruk
Research group leader(s):
Lars Helge Stien (Dyrevelferd)
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Robin Ørnsrud
Norsk sammendrag
Summary
The salmon aquaculture industry currently uses a range of strategies to combat salmon louse epidemics, however acute delousing methods are limited due to poor efficacy, poor welfare and environmental impacts, or potential resistance to medicinal treatments. The three delousing methods most commonly used (thermal, mechanical, and freshwater), used alone or in combination, have not been systematically studied or compared so that the use of these strategies can be properly optimised. This project generated experimental data on delousing efficacy and welfare impacts of single and combined treatments using these three methods, in a tank setting, and found that single freshwater bath treatments and freshwater baths combined with other treatments had the greatest delousing efficacy. The welfare of fish declined in all treatment groups throughout the experiments, and statistical analysis revealed no consistent difference between procedural controls and the individual or combined treatments. The findings suggest that any treatment applied alone or in combination has a negative impact on fish welfare but the handling rather than the treatment itself is the major contributor to poor outcomes. However, the treatments involving a 34 °C warm water bath resulted in the worst welfare outcomes, the lowest condition factors, and lowest growth rates, which suggests that the 34 °C warm water bath is particularly detrimental to fish welfare. The project also investigated the welfare impact of delousing at commercial sites using data acquired from sites over a production cycle. While the management strategies depended more on production goals and regulatory actions, the data also showed that mortality increases after all handling events and delousing operations. The greatest mortality increases were associated with well boat operations including thermal and mechanical treatments. Lastly, the temperature and low salinity tolerance levels, and the ability for these tolerance levels to shift upon exposure, were investigated in larval salmon lice. Three strains were investigated and while their temperature tolerance levels were similar, significant differences in salinity tolerance levels between strains were found. A more detailed investigation at the epigenetic level (specifically targeting 5-mC DNA epimethylation) revealed significant differences between groups, and indicated the potential for salinity tolerance markers to be found in older louse stages. This project provided comparative data on delousing methods can be used to generate knowledge on efficacy and welfare impact on single and combination methods, with various factors taken into account, which should be further verified with procedures at commercial scale.
1 - Background
The salmon aquaculture industry in Norway currently faces challenges in the control and prevention of salmon lice (Lepeophtheirus salmonis) epidemics; recent years has seen a shift away from use of chemotherapeutants towards non-chemical methods, such as delousing with freshwater, mechanical applications or thermal treatments. However, there is not a wealth of data available on the efficacy of these methods, and there has been concern for their negative effects on mortality rates and welfare (Overton et al. 2019), which is greater than the impact of medicinal treatments (Walde et al. 2021). This welfare impact is associated with the stress of handling operations, the treatment itself, and the restriction of feed prior to treatment. The Norwegian Food Safety Authority (Mattilsynet) has recognised this issue and recently stipulated that empirical evidence through experiments and research studies are required to document the welfare consequences of delousing methods and equipment before they are introduced to the market for commercial use (Moore 2021). This would include accompanying guidelines for correct use to ensure welfare status is maintained in fish. Mattilsynet highlights the requirement for testing of non-medicinal methods against lice, and particularly including the combinations of treatments (even if they have been individually investigated). They therefore require documentation of treatment effects from competent and professional sources with expertise in fish welfare, to thus provide quality and reliable evidence before strategies can be approved for commercial use.
This project investigated three common delousing approaches: thermal, freshwater, and mechanical. Thermal delousing using water up to 34 ℃ is the most commonly practiced in Norway, however even the short-term exposure to heated water causes poor post-treatment welfare and elevated mortality (Overton et al. 2019). Salmon have a clear pain-like response during thermal treatments above 28 ℃ (Nilsson et al. 2019), and these results are likely to influence thresholds set by Mattilsynet in the near future. Mechanical delousing generally crowds fish and pumps them through a system that applies brushes or water jets to disturb and detach lice; a technology that used spray jets were very effective at removing mobile lice (Gismervik et al. 2017), however there is scarce published and peer-reviewed evidence for the other technologies available on the market. Early in the deployment of mechanical technologies, farmers surveyed about their perceived impact on the welfare of their fish reported frequent observations of scale loss, gill bleeding, wounds, and increased mortality. Freshwater baths have also been an alternative solution for bath treatments, however concerns for possible resistance development in lice populations has reduced its capacity for commercial use (Ljungfeldt et al. 2017). The uncertainty around the best practice protocol for using these strategies necessitates the improvement and optimisation of methods, so that they are more effective thus requiring fewer delousing events per cage. There is a need for systematic comparison between the single and combined use of methods, to understand how efficacy can be improved whilst maintaining positive fish welfare. Good fish welfare requires the capability of an individual to have a normal, functional cortisol and central nervous system response to stress - this is particularly essential under production contexts where fish are often exposed to repeated stressors and stress events. Hence, analysing not only the physiological state but also the capability to respond to new stressors yields the most complete information on welfare status (Johansen et al. 2020). For example, fish undergoing delousing may not in the prime health condition before they are exposed to acute stress from treatments, and must still be able to mediate their stress response to husbandry activities soon after (such as environmental challenges, harvesting, or repeated delousing due to ineffectiveness). Coping ability to superimposed stressors is not only indicative of welfare status, but can also influence mortality risk and growth in the period after delousing. We used this approach of physiological profiling in assessing welfare to characterise the magnitude of stress experienced by fish after delousing treatments and impact on coping ability.
In addition to success at removing lice, delousing strategies also need to consider the duration of expected effectiveness and potential resistance development in lice, when deciding frequency of delousing method use within a production cycle. Planktonic, infective lice are sensitive to low salinity and will actively avoid brackish water. However, the characteristics of behavioural traits exhibit large heritable variations between families (Coates et al. 2020, Ljungfeldt et al. 2017). This questions the suitability of these factors in preventive strategies since exposure may lead to selection for resilience and subsequent treatment failure. Inheritable traits need not be genetically defined, however, and traits based on epigenetic inheritance is more dynamic and may be lost after a few generations (e.g. Rechavi et al. 2011, Jablonka 2017). This has a large impact on the sustainability of a preventive strategy; if resilience is genetic, and hence quite permanent, a treatment will likely permanently lose its effect over time. In contrast, if resilience is transient and reversible, as is expected for epigenetic traits, it can be overcome by management procedures and the treatment will retain its efficacy. It is expected that adaptation to recurrently changing environmental conditions with a periodicity exceeding that of an individual's generation time is likely to be governed by dynamic epigenetic inheritance (Jablonka 2017). Salmon louse exposure to both low salinities and seasonal high temperatures vary on an intergenerational-timescale and are likely candidates for conditions to which salmon lice will show a transient epigenetic adaptation. This expectation is further strengthened by fact that epigenetically controlled heritable adaptive responses to salinity-challenges have previously been shown in crustacea (Jeremias et. al. 2018) and by preliminary results showing that salmon lice respond to freshwater exposure by changing their global DNA methylation levels. This project investigated the epigenetic mechanism behind variation in low salinity and high temperature tolerance, the potential heritability of these tolerance mechanisms, and whether tolerance to one of these factors is linked to the other, i.e. do lice with high tolerance to temperature also exhibit high tolerance to low salinity.
1.1 - Project information
Declaration of interest
The project was funded by the Norwegian Seafood Research Fund (FHF), who along with the reference group, contributed to discussions around the experimental objectives. They had no involvement in the collection, the analysis or interpretation of data, or in the writing of the conclusions of this report.
The project began in July 2021 and ended in April 2023, including a 3 month extension. The project was led by Samantha Bui (HI), with a 7-month transfer to Frode Oppedal in 2022. The project team from HI included Lars Helge Stien, Jonatan Nilsson, Cameron Thompson, and Angelico Madaro who contributed their expertise in animal welfare (WP1, 2, 4), and Rasmus Skern-Mauritzen, Sussie Dalvin, Takaya Saito, and Kaja Skærven who jointly undertook the epigenetic work (WP3). Professor Øyvind Øverli (NMBU) contributed expertise in stress physiology to WP1, joined by Wayne Korzan (University of West Alabama). The project had constructive contributions from the reference group which included: Kristin Ottersen (Havet AS), Henrik Trengereid (Mowi ASA), Geir Magne Knutsen (Bremnes Seashore AS), and Britt Kari Legård (Eide Fjordbruk AS), plus the FHF moderator Kjell Maroni.
1.2 - Project objectives
The overarching objective of the project was to compare efficacy of single and combined treatments to understand under what conditions do they function best, and how they impact salmon welfare. Secondary objectives were to further investigate this at commercial scale, and to explore epigenetic resistance in lice.
Sub-objectives were to:
-
Determine the individual and combined efficacy of thermal, mechanical, and freshwater delousing strategies on removing sessile and mobile lice
-
Investigate the effect of these strategies on salmon welfare (including individual-based responses)
-
Determine the factors that can positively influence efficacy and welfare
-
To determine the efficacy and welfare impacts of delousing strategies at commercial scale
-
Identify epigenetic C5m DNA methylation markers for high temperature and low salinity resilience
-
Investigate overlap in epigenetic resilience response
2 - WP1 – Delousing efficacy and welfare effect of multiple strategies, at research scale
This experiment was conducted according to the plan presented in the project description, with some adjustments after discussions with the reference group. The objectives for this WP were:
-
Determine the individual and combined efficacy of thermal, mechanical, and freshwater delousing strategies on removing sessile and mobile lice
-
Investigate the effect of these strategies on salmon welfare (including individual-based responses)
-
Determine the factors that can positively influence efficacy and welfare
The WP1 objectives were addressed through three rounds of experiments in which single and combined delousing treatments were applied. Those experiments were carried out in September 2021, March 2022, and June 2022. In addition to those experiments, an add on experiment examined the delousing efficacy of freshwater and hyposaline (ion-modified) water baths over time and their impacts on the physiology of salmon. Those experiments were conducted in January and February 2022. A article covering the round 1 and 2 experiments has been published (Thompson et al. 2023a), and a report describing the freshwater trial has been published under the title “Delousing Efficacy and Physiological Impacts on Atlantic Salmon of Freshwater and Hyposaline Bath Treatments” (Thompson et al. 2023b).
2.1 - Methods
2.1.1 - Experimental Rounds I, II, & III
In the first round of experiments (September 2021), three common garden tanks were used that held 150 fish each, that represented all 15 treatment groups (i.e. 10 replicate fish per group per tank; Table 1). Treatments were either freshwater (FW) baths for ~20 hours (length due to logistical constraints), thermal bath for 30s at 28 or 34 °C, mechanical delousing (applied to all sides of the fish at 2 bar pressure), or a combination of these conducted in this order (Table 1, Fig. 1). An additional investigation into the factors influencing the efficacy and welfare outcomes of these non-medicinal treatments was conducted through two additional rounds of experiments. Here, two rearing temperatures were examined, one round at 8 °C and a lower temperature round at 6 °C, with both larger and smaller fish of approximately 5 and 2 kg included in the experiments. Each round of experiments had 6 tanks each with 70 fish held in a common garden design (n=420). These rounds of experiments were conducted in the same tanks as the first and separated by the temperature regime, with the 8 °C temperature experiment conducted in March 2022 and the lower 6 °C temperature experiment in June 2022. In both sets of those experiments treatments were carried out in 6 combinations along with a negative control. As in the first round of experiments, these treatments included the two warm water baths of 28°C or 34 °C, a freshwater bath of 4 hours, and a mechanical delousing treatment. In half of the treatment groups, the salmon were also sedated prior to treatment with warm water or mechanical delousing.
In all experiments, fish were individually tagged, and infected with sessile, pre-adult, and adult lice, then delousing treatments applied according to the individuals’ treatment groups. Lice were counted on a subsample of fish in each tank prior to treatments. After treatment, fish were returned to their original tanks. The whole tank was sampled either 24h post-treatment or 3 weeks post-treatment, for body size, lice abundance (including all stages), welfare status (using an adapted form of both FISHWELL and LAKSVEL), and blood plasma. In the first round of experiments an additional physiological stress test was conducted in which a subset of fish were collected 3 weeks after treatment, confined for 2 hours to instigate a stress response, and then sampled for blood plasma cortisol and brain serotonin production. Rather than measure serotonin directly, the ratio of two brain metabolites are used as a biological marker of its production: 5-hydroxyindoleacetic acid (5-HIAA) and its precursor 5-hydroxytryptamine (5-HT).
A malfunction during the March 2022 experiments prevented the recording of the individual PIT tags used to track the fish and thus most of the observations of fish welfare and lice load could not be associated with their treatment group. However, data was collected from two of these tanks, including a set of pre-treatment observations from one of them which enables analysis of delousing efficacy. The June 2022 round of experiments with the lower temperature also encountered circumstances that resulted in the loss of 3 experimental tanks holding the smaller size class of salmon. Prior to the experiment start, the welfare of those fish deteriorated, and they were euthanized to prevent any further suffering. The welfare condition of the larger salmon was better and the experiment could therefore continue with these fish as planned.
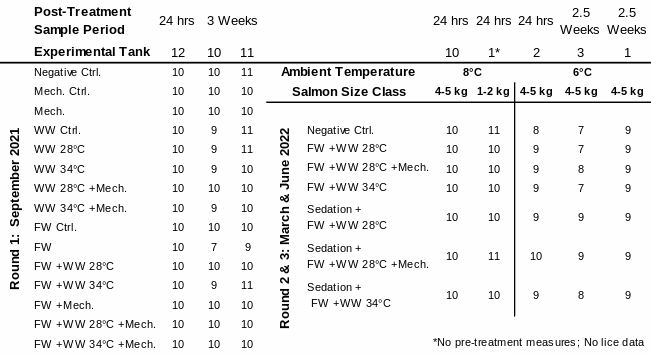
Table 1. Distribution of experimental fish: sampled number of salmon in each treatment group by their treatment tank, size class, ambient temperature, and experimental round. Warm Water (WW); Freshwater (FW); Mechanical (Mech.); Control (Ctrl.).

2.1.2 - Efficacy of freshwater and hyposaline bath treatments at various durations
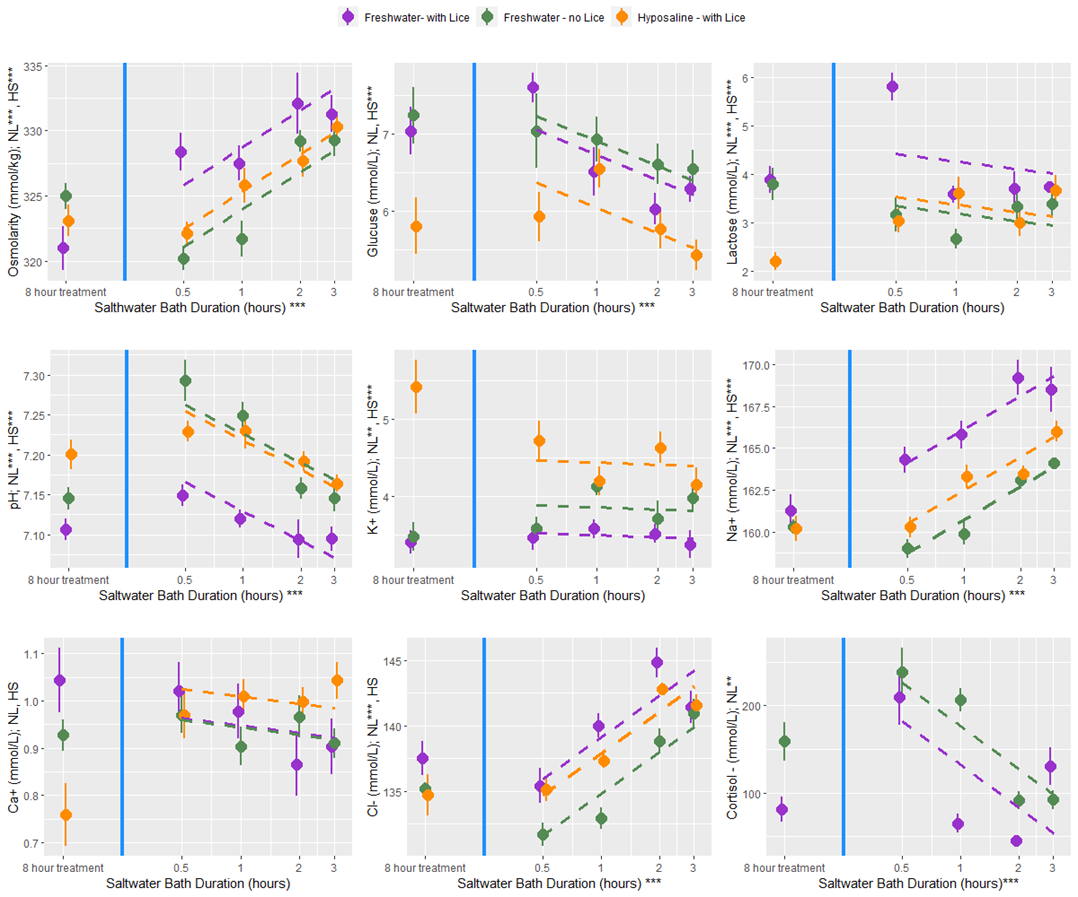
An additional experiment was conducted to investigate the delousing efficacy of freshwater and hyposaline (ion-modified) water for various treatment durations. While the experimental setup differed from the other rounds, the handling and sampling procedures were nearly the same and produced comparable datasets. There were four primary treatment groups: infected fish held in seawater (procedural controls), infected fish treated in freshwater (buffered with 1% seawater), uninfected fish treated in freshwater, and infected fish treated with hyposaline water. This ion-modified water was created using patent-pending nano-filtration technology that essentially creates desalinated seawater (Fiizk AkvaFresh AS). In groups of 10 fish were held for a period ranging from 30 minutes to 48 hours in their designated treatment bath. Fish were then netted into a separate vessel with sedatives, and once unconscious, the number of lice for each fish was enumerated, measurements for weight and length were taken, and blood samples were collected. The salmon were then transferred through a live-fish pump to simulate treatment conditions at a farm, and the louse number was counted again to determine the proportion loss associated with the mechanical disturbance. In another secondary experiment aiming to characterize physiology, a subset of fish from the fresh and hyposaline water treatment groups had their treatment water switched to seawater after the initial 8-hour bath and were held for an additional 30 minutes to 3 hours.
This experiment was conducted according to the regulations on use of animals in experimentation, and approved by the Norwegian Food Safety Authory (application ID 27999).
2.2 - Results
2.2.1 - Delousing efficiency Round I experiment
Mobile lice only – 24h post-treatment results: The mean number of mobile lice per fish in Tank 12 was 10.9 prior to treatment. For immediate removal of mobile lice, methods that included thermal (34°C) or freshwater treatments were most efficient, with close to 100% removal (Fig. 2). Their effect was relatively similar among these treatments, with little apparently effect of mechanical treatments. Because almost all lice were removed, whether or not the combination of freshwater and thermal 34°C would have had an even higher efficacy than individual methods is unknown. This pattern was similar if comparing the total number of lice (including sessile stages) before and after treatment (Fig. 2).
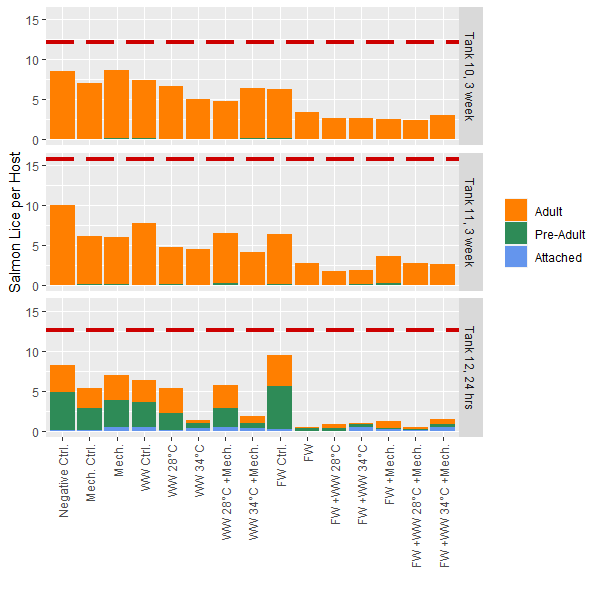
Total lice levels – 3 weeks post-treatment results: The mean total lice numbers in Tank 10 and 11 were 12 and 16 lice per fish prior to treatments, respectively. Contrastingly to results for 24h post-treatment (Tank 12), the long-term delousing efficacy appears highest in any treatment that included freshwater bathing, while Thermal 34°C was similar in efficacy to Thermal 28°C (Fig. 2). This is logical if sessile stages are killed by FW exposure, and therefore do not further develop during the subsequent 3 weeks, while if sessile stages survive thermal treatments they can continue on the host and be counted at the 3w sample. There also appears to be reasonable loss associated with handling and moving fish, as seen through the procedural control groups (Control FW, control mechanical, control thermal) compared to the negative control fish were minimally handled (Fig. 2). As this was a common garden experiment it is also possible that mobile lice could move between fish in the tanks prior to sampling.
2.2.2 - Delousing efficiency Round II & III Experiment
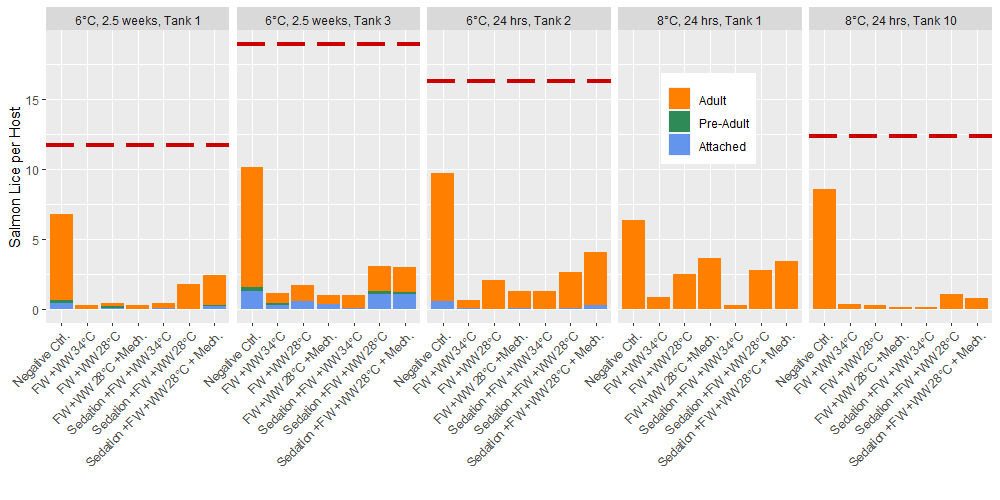
Prior to delousing treatments, the total lice loads in experimental tanks ranged 11.7 to 18.9 lice per fish with 100% of the lice in the 2nd round experiments (8 °C) being adult stages, and 3.4% of the 3rd round experiments (6 °C) being attached chalimus stages. After application of the delousing treatments in these rounds of experiments the total lice in the experimental tanks was found to range from 4% to 12% of the pretreatment lice loads if the negative control group is removed. Within the negative control group the lice load was 53% to 70% of the pre-treatment level (Fig. 3). All treatment groups apart from the negative control included a freshwater bath treatment and the treatment effects seen here are comparable to the freshwater bath effect seen in the first round of experiments 24 hours after delousing (Fig. 2). Only adult lice were found in the post treatment lice counts of the round 2 (8 °C) experiments, but 6% to 24% of the lice in the round 3 tanks (6 °C) were in the attached chalimus II stage, and up to 4% were preadults. In the round 3 experiments (6 °C), the post-treatment counts of lice occurred 4 and 24 days after the pre-treatments counts which is predicted to result in 0.14 to 1.34 molts at the experimental temperature (Hamre et al. 2019). Thus, in the tanks sampled 2.5 weeks after treatment the pre-adults likely developed from chalimus in the time since the pre-treatment observation, but it is unlikely that any chalimus II had developed from newly attached copepodids. Therefore, the delousing treatments had a greater effect on the mobile stages since the relative proportion of attached stages increased.
2.2.3 - Statistical Analysis of Delousing Efficacy.
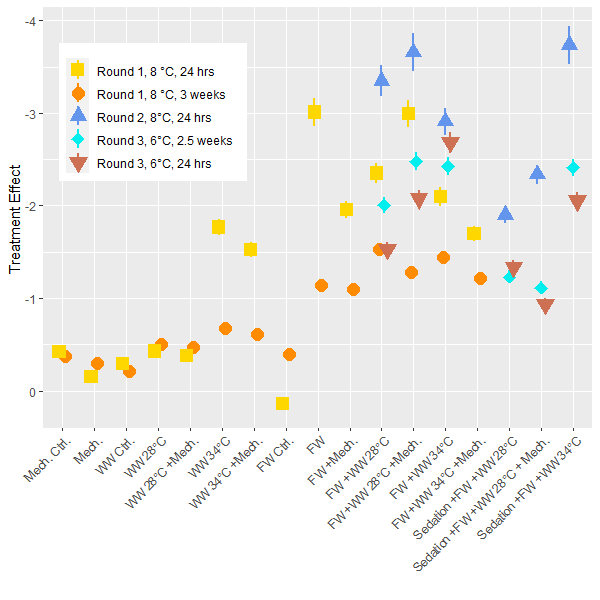
The lice load per fish was analyzed for each experimental round and post treatment sampling period to determine the delousing effect. In these generalized linear models the pre-treatment lice load is considered as well as the size of the fish and differences between tanks. The model then determines the total delousing effect of each treatment while accounting for these other factors. Direct comparisons are best made within each experiment, but since the experiments all followed the same setup the results are comparable. Overall, the freshwater treatment is confirmed to be the most effective at overall delousing and the freshwater treatments combined with other treatments are just as good with some a little better (Fig. 4). The models suggest that sedation decreases the efficacy of treatments but the effect for these treatment groups is still greater than those without a freshwater bath. The efficacy of treatments is lower for the 3rd round of experiments conducted at the lower ambient temperature of 6 °C compared to the experiments conducted at 8°C. Although it is not a large temperature change, it does suggest that the Δ T is not a driving factor here, or if there is a Δ T effect it is not observable when a freshwater treatment is primarily responsible for delousing. In the models the size of the fish was often associated with an increase in lice load which conforms to earlier findings in the literature which describe how larger fish are infested with more lice. Otherwise, in the range of sizes seen here (1 – 5 kg) there is no interaction between delousing efficacy and fish size.
2.2.4 - Welfare Status (prevalence of external injuries)
Across experimental rounds the prevalence of injuries is higher in the post-treatment datasets than in the pre-treatment samples (Fig. 5). In all experimental rounds, Fisher exact tests indicated no significant differences between tanks in the Pre-treatment samples for each welfare category. Overall, the post treatment fish in the 3rd round of experiments at 6°C had a greater prevalence of injury in comparison to the earlier rounds, with fish from the second round 8°C temperature round having the lowest prevalence of injury. Specifically, after treatment application 70% of fish in round 1 experiments had at least one injury, 64% of fish in round 2 had at least one injury, and 97% of fish in round 3 had at least one injury. Thus, injuries were widely prevalent in the experimental fish and assessing the impact of treatments was done through statistical modeling according to treatment groups. In the post-treatment datasets from all experimental rounds, the statistical models indicated that delousing treatment, fish attributes (length, weight, or condition factor), and experimental tanks significantly predicted an injury outcome just 8% of the time. Furthermore, only in the round 1 experiments sampled at 3 weeks did any of the models show that a treatment significantly predicted an injury outcome (the maximal treatment combination with 34°C thermal bath). Considering the number of statistical models selected we can expect a significant outcome in some of them due to type I errors. Thus, the overall analysis suggests that delousing treatments do not predict more injuries than their treatment controls, but the controls also have injuries. Overall, the welfare outcomes of experimental fish were poor (Fig. 5), but delousing treatment appears to have no effect on the prevalence of injuries as assessed by FISHWELL/LAKSVEL scoring.
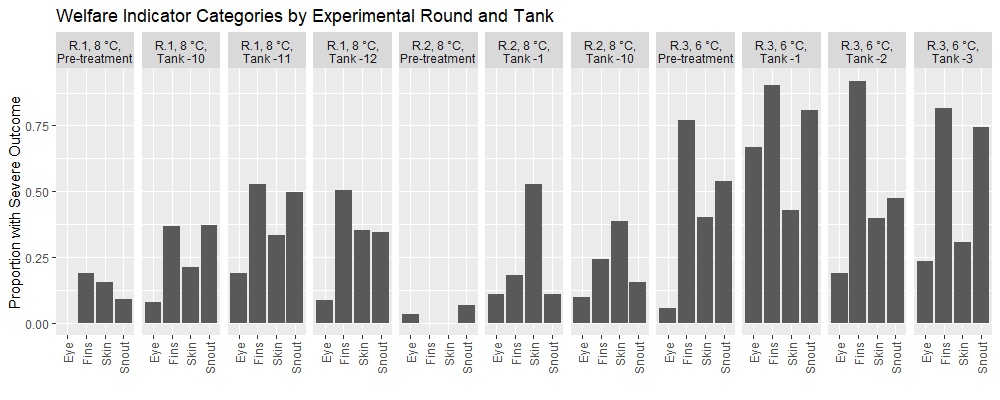
2.2.5 - Growth and condition factor
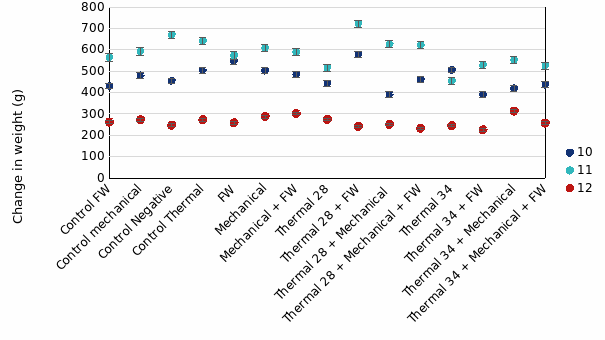
In the 1st round of experiments, fish treated then sampled 24-hours post-treatment were very similar in weight among treatment groups compared to their size 35 days prior (Tank 12 in Fig. 6). For fish sampled at 3w post-treatment, mean weight gain over the 53 days was 471g and 587g for Tank 10 and 11, respectively. There was relatively similar growth between treatment groups, with a slight trend of fish exposed to Thermal 28°C treatments gaining slightly more weight than those exposed to Thermal 34°C (Fig. 6) in Tank 11. This was not the case in Tank 10.
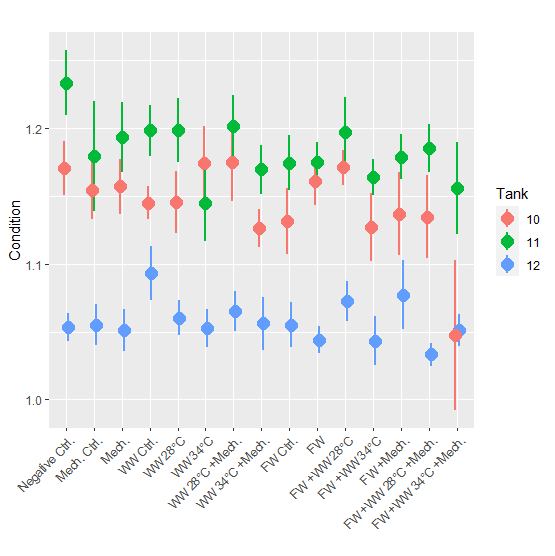
Condition factor for the 24-hour post treatment (mean = 1.1) was lower than for the 3-week post treatment groups (mean = 1.1 and 1.2 for Tanks 10 and 11, respectively) (Fig. 7). When the 3-week post treatment dataset was examined with a statistical model it was found that 3 of the 4 treatment groups with the 34 °C bath had a significantly lower condition factor than the negative control. The freshwater control group also had a significantly lower condition factor, but not any other group. In the 2nd and 3rd round of experiments length and weight was not measured during PIT tagging and growth could not be calculated. In the 3rd round of experiments where fish were sampled 2.5 weeks after treatment, the condition factor was examined but no significant differences were found between treatment groups.
2.2.6 - Blood parameters
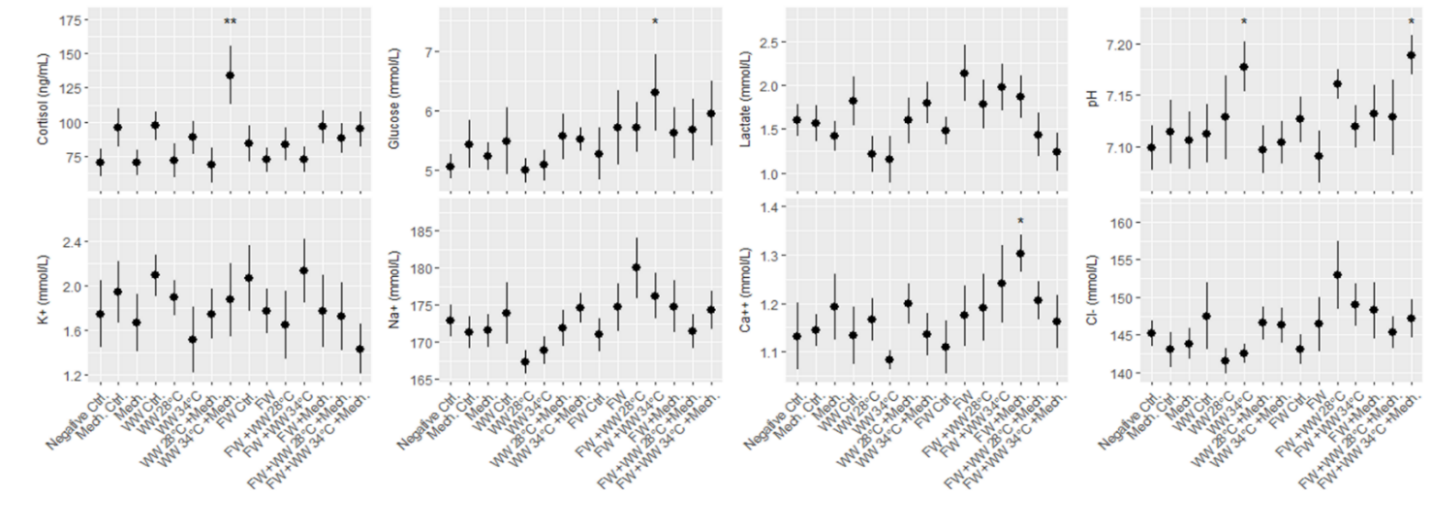
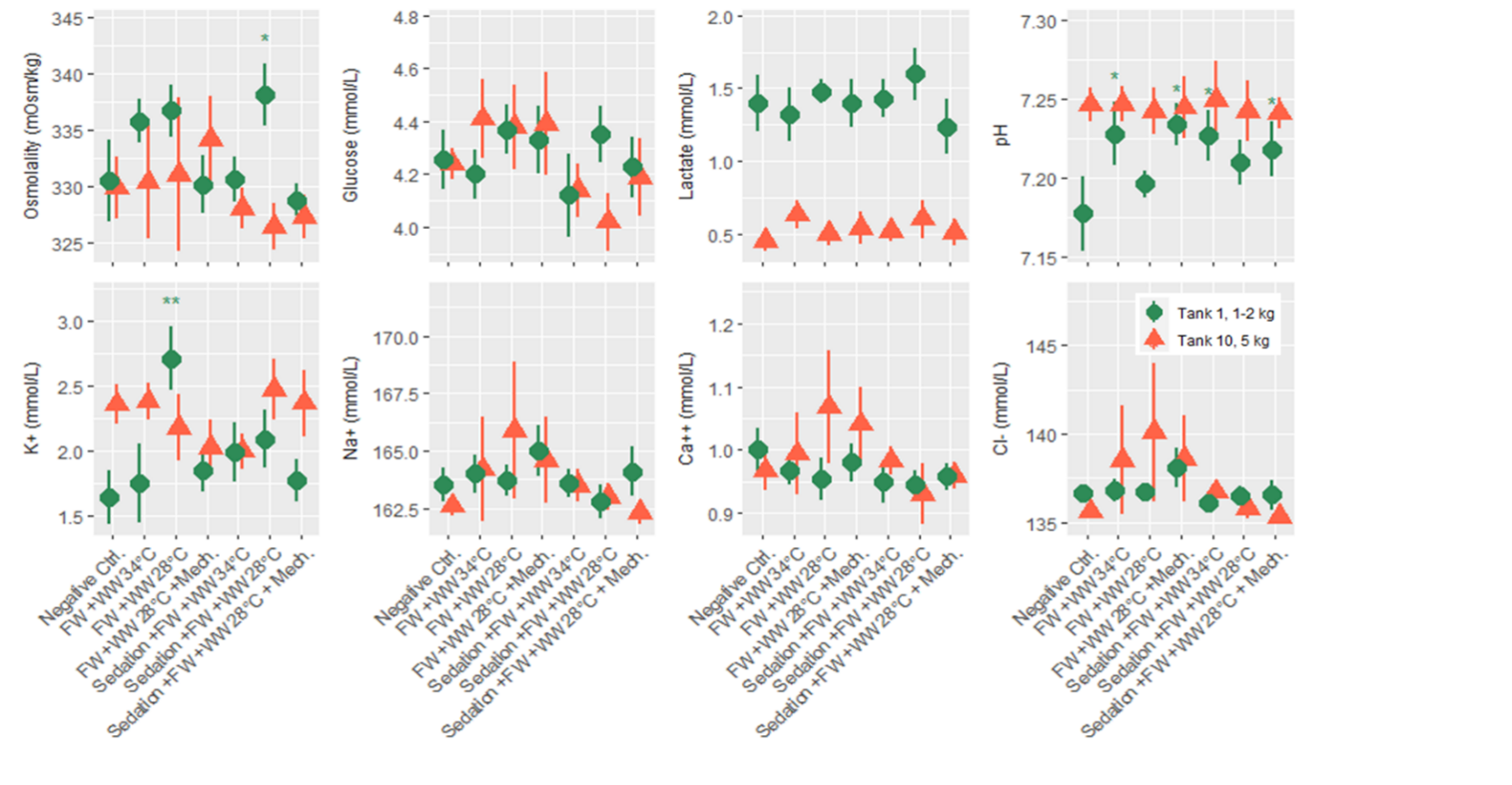
Salmon from each of the three rounds of experiments were sampled for blood 24 hours after treatment, and 7 plasma parameters and either cortisol or osmolality was measured. According to statistical models the order of blood sampling significantly effected the measurements of cortisol and pH in the first round of experiments. Otherwise the linear models detected a few significant differences from the negative control treatment, specifically the freshwater and 34 °C warm water bath delousing was associated with a higher concentration of glucose (Fig. 8). In the second round of experiments blood sampling order significantly effected the measurements of pH in both the large and smaller size class of fish, and it effected potassium and sodium in the small size class. Significant differences from the negative control treatment were only found in the small size class, with pH being greater in a number of treatments and two treatments in osmolality and potassium datasets having significantly greater measurements. Across the parameters it is notable that lactate in the smaller fish (1-2 kg) is greater than in the large fish for all treatment groups (Fig. 9). Generally, these treatments appeared to be stressful through the handling and processing of fish rather than the treatments themselves, as treatment groups had similar responses to the procedural controls. It is also important to note that stress response is typically greatest in the first few hours after the animal encounters the stimulus but here we are examining stress 24 hours after the incident by looking at cortisol, pH, glucose and lactate. Furthermore, the impact of delousing treatments on osmotic balance is not well described but it is understood that a fish transferred to freshwater will both lose ions from its blood plasma and take in water. The dataset here suggests that the delousing treatments did not have an impact on the osmotic balance of the fish, or that the animal was able to recover its osmotic balance within 24 hours which is likely for the freshwater treated fish.
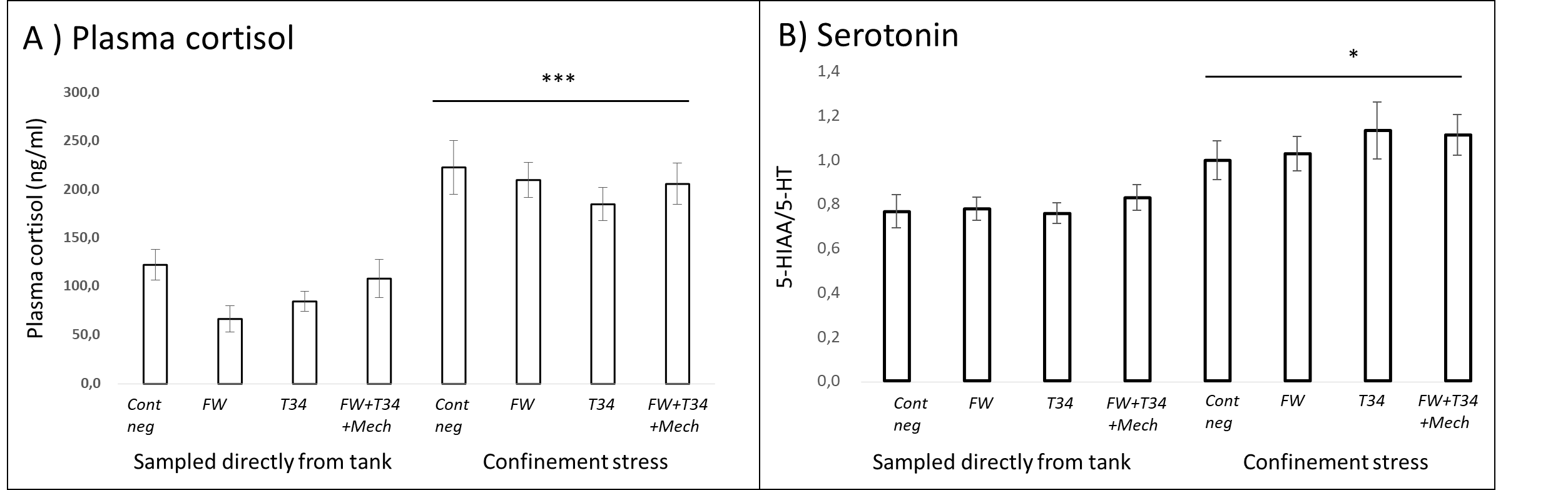
2.2.7 - Confinement Stress Response
Fish from all treatment groups which were subjected to the additional confinement stress exhibited elevated blood plasma cortisol and serotonin production in the brain (Fig. 10). The result indicates that all the treated fish were able to respond appropriately to a new stressor. Had the treatment or experimental conditions induced a chronic activation of the stress response in the fish, their ability to respond to an additional stressor would have been reduced. Thus, neuroendocrine indicators suggest that potential negative welfare effects of delousing are temporary and reversible, regardless of treatment.
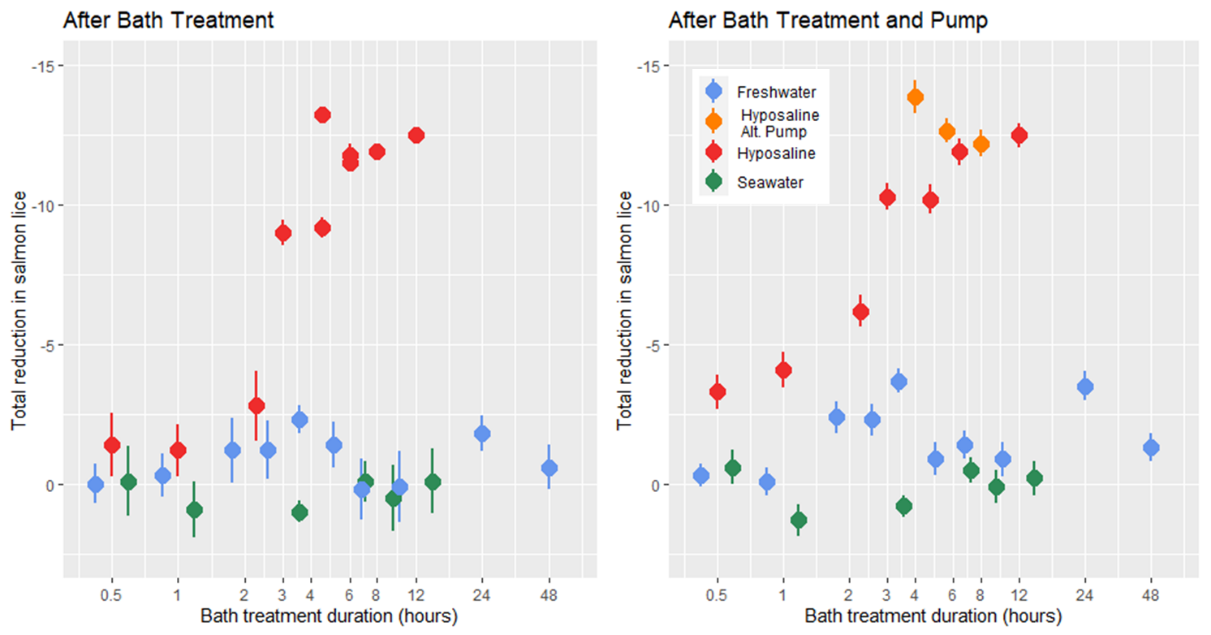
2.2.8 - Hyposaline and Freshwater Trial
Prior to treatment all lice were in the adult stages and the mean lice load of all infected salmon was 9 .37 ± 0.29, with the mean load of individual treatment tanks of 10 fish (n = 28) ranging from 3.6 to 15. At the time of treatment application the mean salmon weight was 355.7 ± 4.3 g, length was 32.2 ± 0.5 cm, and condition factor was 1.09 ± 0.01. In all bath treatments there was a decrease in lice numbers from before the pump transfer to after, but according to statistical models the freshwater treatment had no delousing effect (Fig. 11). The hyposaline bath treatment did have a significant effect on the number of lice observed after the bath and after the pump transfer with the lice load decreasing with bath duration. The greatest relative reduction in lice load for the hyposaline treatment happened between 2 and 3 hours in the bath. After 6 hours in the post-bath counts and just 4 hours in the post-pump counts the lice load was less than 5% of the pre-treatment levels (Fig. 11). Although the freshwater treatment was not found to predict a lower lice level in the models, the lice load after pumping is lower in the freshwater treatments in comparison to the seawater treatments. Overall, the lice counts after the freshwater bath were 88% that of the pre-treatment levels, and after pumping the lice counts were 76% that of the pre-treatments.
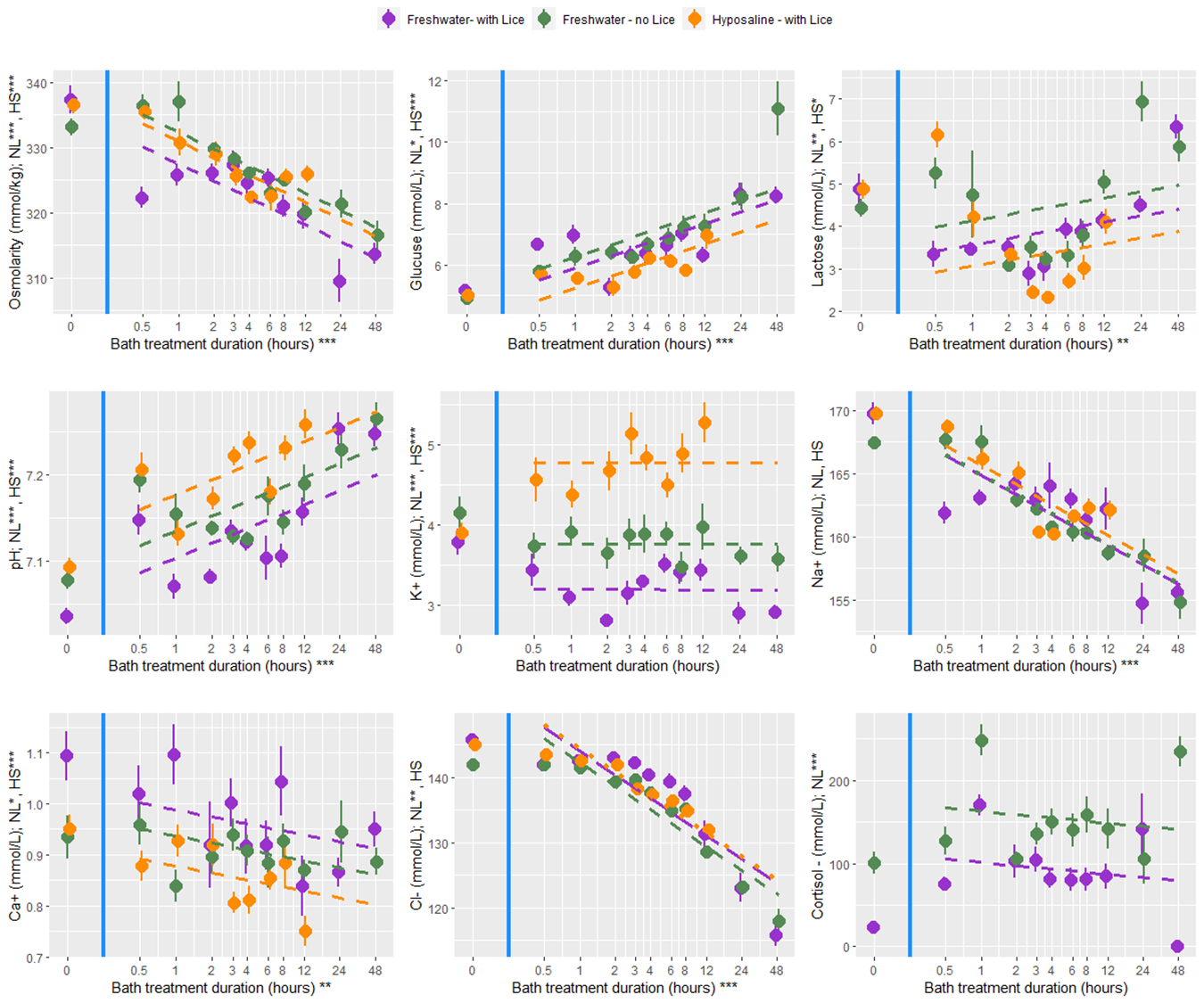
At all bath treatment durations there were 9 blood plasma parameters measured from louse infected and uninfected fish treated with a freshwater bath and from infected fish treated with a hyposaline bath. While there are statistical differences between the treatment groups the overall finding is that both freshwater and hyposaline bath treatments are stressful and lead to osmotic imbalances (Fig. 12). Furthermore, the stress and osmotic disruption increases with bath duration which supports the general recommendation that the bath duration should be no longer than necessary. When fish are moved from an 8-hour bath treatment back to seawater their stress decreases (cortisol, glucose, and lactate) and their osmotic balance returns towards normal (osmolarity and ions) (Fig. 13). That dataset here is valuable in its description of these physiological processes which are elsewhere undescribed, particularly for infected salmonids. However, caution should be exercised when making broader inferences about the treatment effects because there are no replicates and the experiments were conducted over two experimental rounds in January and February 2022.
3 - WP2 – Delousing efficiency and welfare effect of control methods, at commercial scale
3.1 - Methods
Commercial production data was analyzed to evaluate fish welfare, fish mortality and delousing efficacy depending on the treatment applied and other influencing factors. The data was collected and organized as part of a project to document the health and welfare of triploid salmon produced under “green licenses” which were issued to NRS Farming AS and Wilsgård Fisekeoppdrett AS in 2014. Collaborators in the project included the farm companies, the fish health services, and the Institute of Marine Research which has published reports for each of the production generations monitored. For the 2020 generation 16 fish groups comprising more than 2 million fish were tracked throughout their production cycles. A full description of the methods and results can be found in the report by Stien et al. (2023), here we briefly present those parts that are most relevant to the OptiDelouse project.
The commercial production dataset contains information on the location and origin of the fish including the specific fish cage they are held in, the hatchery they came from, their transfer to sea date, ploidy, and strain. The dataset has either daily or weekly observations of fish number, weight, mortality, external injuries, number of lice (sessile, mobile, adult females), and sea temperature. All treatment and transfer events are recorded with parameters noting the treatment applied (e.g. AlphaMax, Hydrolicer), and the specifics of the application such as the use of a well boat or tarpaulin, the application duration, treatment temperature (for warm water baths), dosage amount, and number of fasting days prior to the handling event. In addition, the overall health and production trajectory of each fish group was determined by evaluating the monthly reports prepared by the fish health service and by interviewing the farmers. This reporting includes a mixture of qualitative and clinical data which provides an insight into the health situation at each farm and insight into the husbandry decisions made by the farmers. For the purposes of the OptiDelouse project, specific analyses were made on treatment efficacy and welfare impact, but the overall findings of the investigation provided important context for those findings.
3.2 - Results
The investigation found that farmers make husbandry decisions with the aid of fish health personnel that balances the welfare needs of the fish with their production goals. However, the scope of possible actions is constrained by regulations, and due to the lice limit rules farmers can be forced to make decisions that have negative impacts on the welfare of the fish and the production outcomes (see Macauley et al. 2022). It is well established and understood by both farmers and fish health personnel that the delousing operations are harmful to the salmon and a major driver of increased mortality and poor welfare outcomes. Furthermore, it is recognized that the non-medicinal treatments have a greater negative impact on the fish than chemotherapeutics but due to resistance those treatments are less effective (see Overton et al. 2019, Grefsrud et al. 2022). These regulatory constraints intersecting with the opposing welfare and production goals has resulted in the emergence of a number of repeated decisions made by the farmers: 1) farmers choose riskier operations for those fish perceived to be stronger and reserve gentler operations for those that are perceived to be weaker; 2) farmers seek to limit the number of delousing operations to limit welfare impacts and 3) farmers seek to maximize the time between treatments to allow for recovery; 4) riskier operations are preferentially conducted when temperatures are moderate rather than cold so that wound healing is encouraged; 5) longer fasting time is used prior to riskier operations in an attempt to reduce to impact on fish. The matrix of dependent husbandry decisions confounds analyses of the data set which is further complicated by the variation in fish between groups due to their divergent origins. This complex system of decision making is important to understand for drawing unbiased findings from the commercial dataset, and for its implications for optimizing the welfare outcomes and efficacy of delousing treatments. A full description of the findings can be found in the report; these brief summaries describe those most relevant to this project’s objectives.
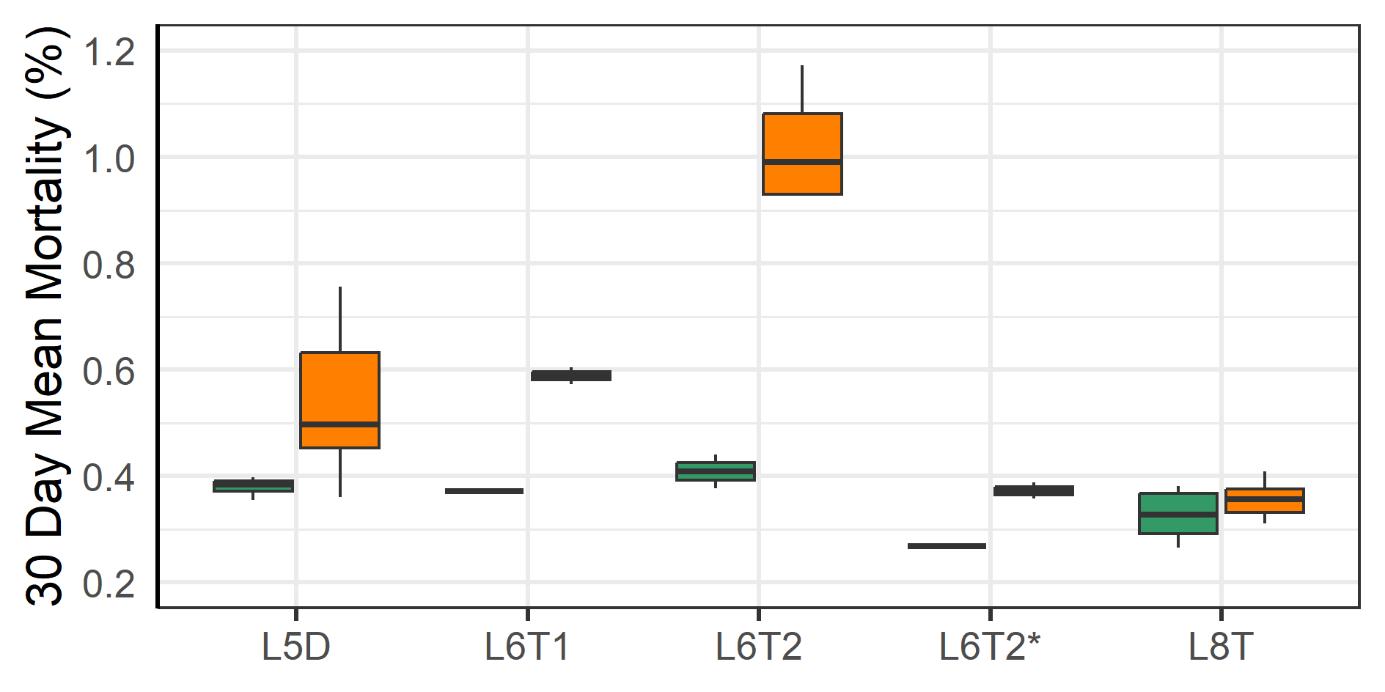
3.2.1 - Mortality and Welfare Consequences of Moving Fish to New Farm
Following the movement of fish from one farm to another the mortality increased at those farms (Fig. 14). The moving operations required the crowding of fish, pumping them into a well boat, transporting them and pumping them into a new sea cage. Those steps are known to stress fish and increase the risk of harm to fish (Overton et al. 2019, Bang Jensen et al. 2020, Oliveira et al. 2021, Walde et al. 2021, Persson et al. 2022). Moving operations done under low sea temperatures (4-6°C) were associated with higher rates of mortality than those done under more moderate temperatures (9°C). That difference could be attributed to a lesser ability of the fish to cope with stress at low temperatures and to the higher prevalence of wounded individuals in the coldwater group. After the moves the prevalence of wounds decreased which suggests that those individuals were the ones that died.
3.2.2 - Lice Load and Mortality Before vs After Delousing Operation
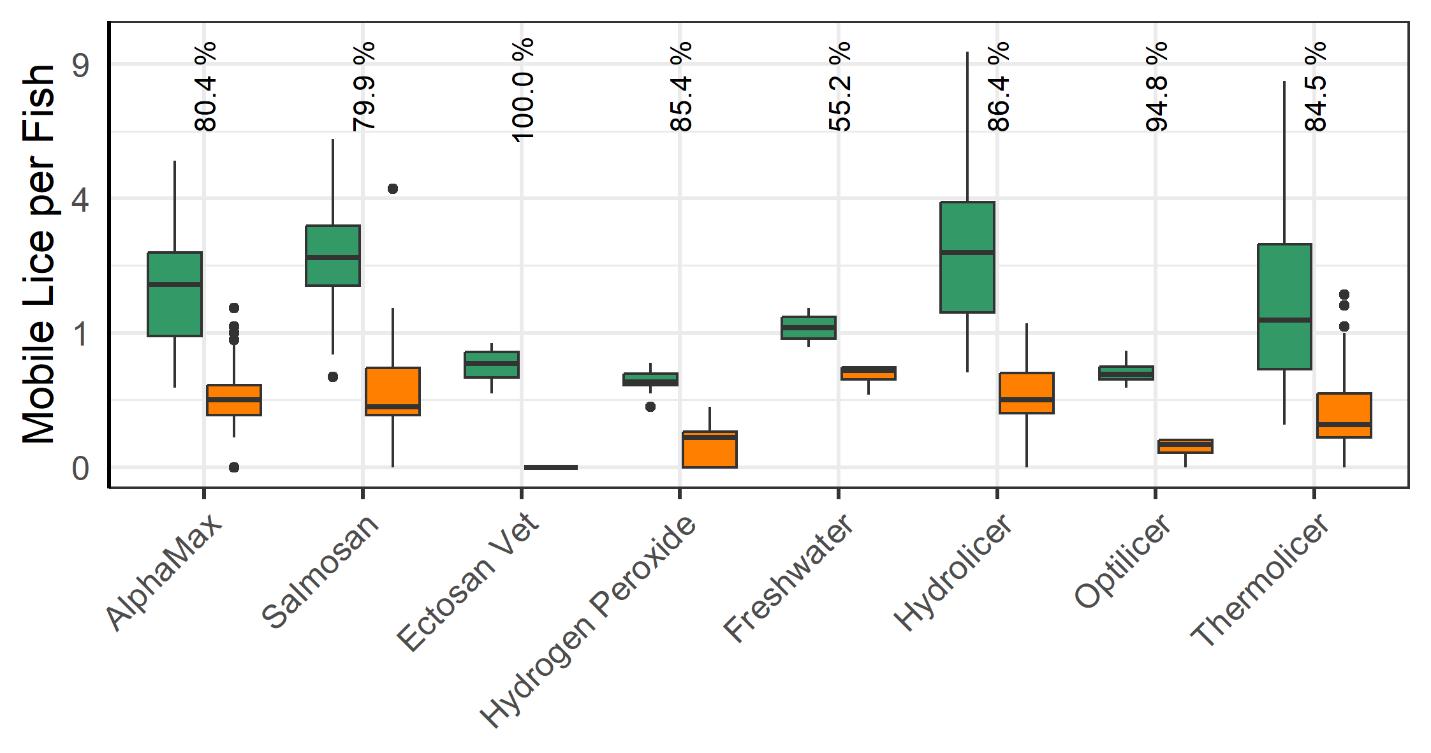
All delousing treatment methods lead to a significant reduction in mobile lice (Fig. 15). The most effective method, Ectosan Vet, is a newly developed chemotherapeutic applied in a well boat. However, it was used on several cages at a single location and caution should be exercised in interpreting the result. Likewise, the freshwater treatment showed the least effectiveness, but many factors can influence its application effectiveness, and while freshwater treatment has demonstrated effectiveness against attached stages (see Powell et al., 2015, Section 2) the count data for the attached stages here was deemed too unreliable for analysis. The medicinal treatments of AlphaMax and Salmosan, along with the mechanical Hydrolicer and warm water Thermolicer treatments all had similar efficacies in removing mobile stages of lice.
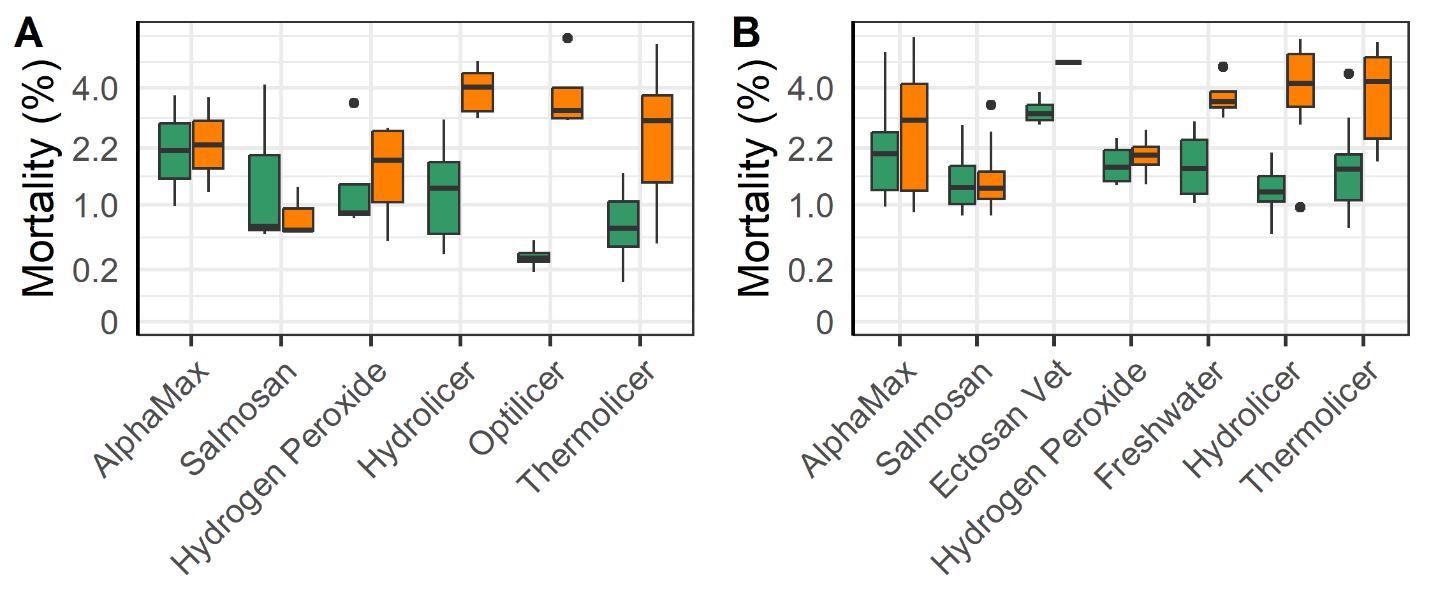
The mechanical and thermal treatments were associated with a greater increase in post vs pre-treatment mortality compared to the legacy chemical methods (Alphamax and Salmosan; Fig. 16). Those treatments along with the application of freshwater baths and hydrogen peroxide use tarpaulins to concentrate the fish within their cage rather than using well boats. The added handling steps required to treat fish in a well boat may account for some of the increased mortality of mechanical and thermal treatments, as well as Ectosan Vet. According to linear regression models the triploid fish had a somewhat greater increase in mortality following treatment compared to the diploids despite the efforts of farmers to mitigate impacts on these perceived weaker fish (Fig. 16).
4 - WP3 – Epigenetic resilience to delousing methods
4.1 - Overview
The aim of WP3 was to investigate if exposure of copepodids to reduced salinities or high temperatures increase resilience towards these treatments in subsequent generations through epigenetic DNA methylation. This was done in two steps: First the sensitivities of established laboratory strains were determined (see section 4.1.1 and 4.1.2) and then the effect of copepodid exposures in two selected strains (see. section 4.1.3) were monitored in the following generation (see sections 4.1.4 and 4.1.5). The overall design was as follows: Copepods were obtained from the Ls1a, LsGulen and LsOslo strains for determination of their resilience towards respectively high temperatures and low salinities. Their survival, activity and total 5-methylcytosine levels were measured using Elisa kits to detect response profiles. Based on the resilience profiles two strains were selected for subsequent investigation of the inheritability of these properties and the underlying epigenetic mechanisms.
4.1.1 - Determination of salinity resilience
Copepodids from Ls1a, LsGulen and LsOslo strains, cultured at 10 °C and full seawater salinity, were synchronized to be 9.5 +/- 0.5 days post-hatch when entering the experiment. Copepodids in triplicate batches (one egg-string each for each treatment parallel) were transferred stepwise to the desired salinity where they remained for 120 minutes (Fig. 17A, B) . This was done by transferring them to consecutively lower salinities in 4ppt steps allowing 5 minutes for acclimatization at each step. After treatment the process was reversed and the larvae were transferred stepwise back to full salinity. After ~18 hrs (i.e. the next morning) the animals’ activity was scored on a scale from 1-5, where 1 indicated that all copepodids were dead and 5 indicated that all were in perfect condition and actively swimming. The intermediate scores should be interpreted as follows: 2 –less than half of the copepodids were active; 3 – approximately half of the copepodids were active; and 4- more than half of the copepodids were active. The activity scores were analyzed with generalized additive models (GAM) following an ordinal response distribution to investigate possible differences between strains and to determine salinity thresholds for activity. After activity was scored the copepodids were preserved in EtOH and the global methylation levels were analyzed with 5-mC DNA ELISA Kit from Zymogen according to the manufacturer’s instructions. This analysis indicated copepodid methylation levels to be very low - and in the very low range of the kits level of sensitivity (data not shown).

4.1.2 - Determination of temperature resilience
Copepodids from Ls1a, LsGulen and LsOslo strains, cultured at 10 °C and full seawater salinity, were synchronized to be 9.5 +/- 0.5 days post-hatch for the experiment. Copepodids in duplicates (one egg-string each for each treatment parallel) were transferred from the incubators to the treatment and left there for 10 minutes (Fig. 17C). Thereafter the wells were returned to the intake water. After ~18 hrs (i.e. the next morning) the animals’ activities were scored on a scale from 1-5, as described above. As with the salinity dataset the temperature dataset was analyzed with GAMs to investigate phenotypic differences between strains. The copepodids were subsequently preserved in EtOH and the global methylation levels were analyzed with 5-mC DNA ELISA Kit from Zymogen according to the manufacturer’s instructions. As described in section 4.1.1 copepodid methylation levels were almost undetectable at the kits level of sensitivity (data not shown).
4.1.3 - Statistical methods
Data from the salinity and temperature resilience experiments were investigated with statistical models in four parts which investigated differences in tolerance due to salmon louse strain and due to parental exposure. In all cases the datasets were fit to generalized additive models (GAM) using the ‘mgcv’ package in the R programing software. Hypothesis testing was done by examining model coefficients and by comparing contrasting models using the likelihood ratio test (LRT). In the GAMs a smoothing term was fit to salinity or temperature exposure level as it relates to activity. Since the response variable activity was categorical with 5 levels, the distribution family selected was ordinal and the models used the logit link function. Thus, the model outputs presented in the figures represent the probability of being in an activity category given the exposure level, louse strain and treatment group.
4.1.4 - Selection of strains, treatment, and fish infection for inheritance determination
The results showed similar tolerance levels for all three strains for both salinity and temperature (Figs. 18, 19, 20). Further details are given below and explain why the LsOslofjord and Ls1a strains were chosen for the inheritance study since: A) The LsOslofjord appeared to show a more gradual increase in activity with salinity which suggests a potential for a phenotype shift. B) The Ls1a the strain for which the salmon louse genome has been sequenced (Skern-Mauritzen et al. 2021).
Analysis of P0 salinity sensitivity.
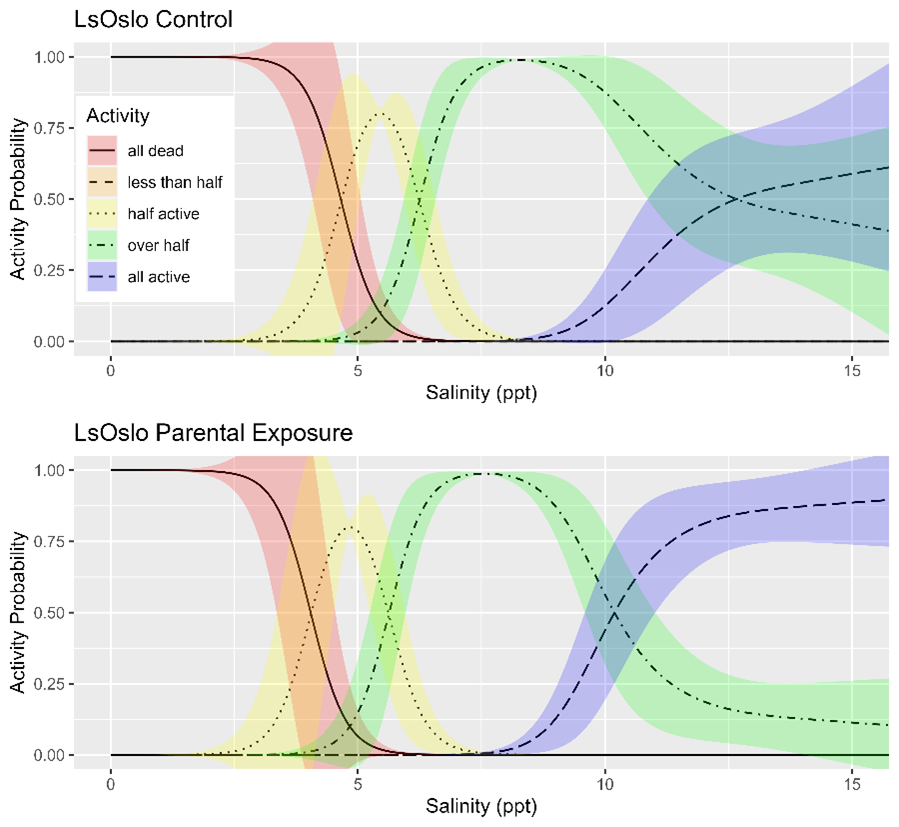
Salinity tolerance was observed to be similar across all three strains of salmon lice with the activity category rapidly increasing from all dead to all active between the lowest salinity of 2 ppt to a salinity of 14 ppt (Fig. 18). However, when the dataset was fit with GAMs it was found that a model with strain as an explanatory variable better predicted activity level than one without it (LRT, p=0.028). The selected model further indicated that at any given salinity level the LsOslo strain was significantly less active than the contrasting Ls1a strain (p=0.048). The LsOslo strain was also less active than the LsGulen strain, but not significantly so (p=0.055). Compared to the other strains, copepods from the LsOslo strain had a higher probability of being categorized as over half active and a lower probability of being categorized as all active at salinities greater than 8 ppt (Fig. 19).
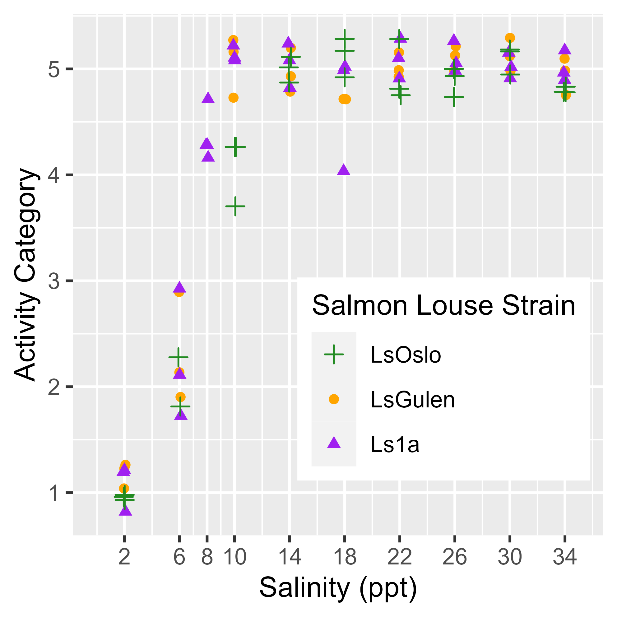
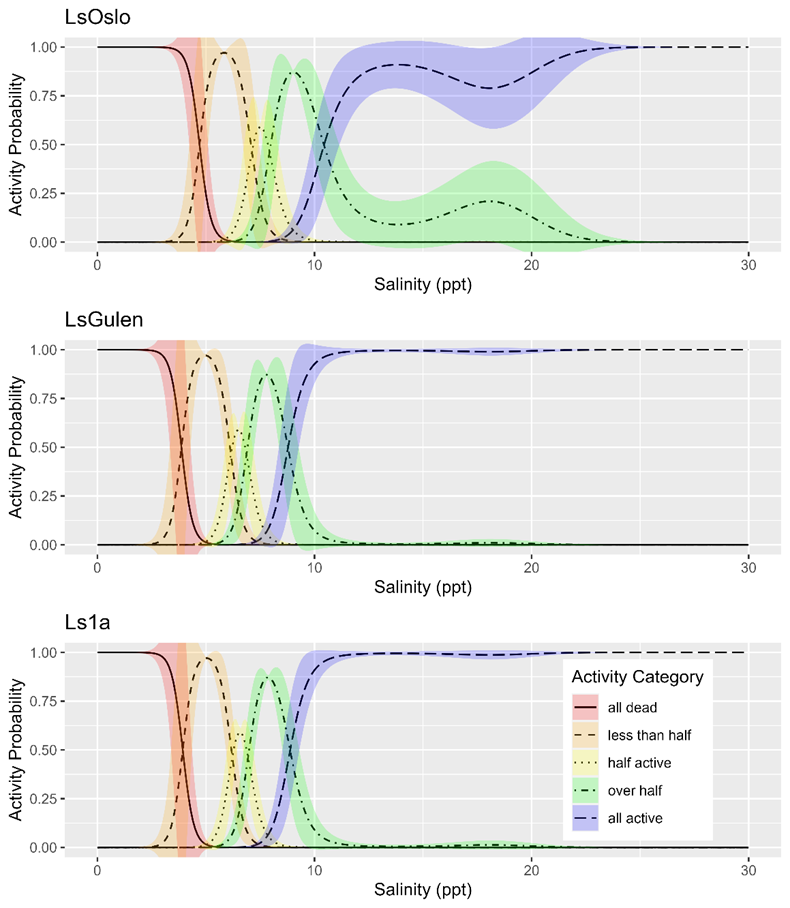
Analysis of P0 temperature sensitivity.
The observed activity of copepods in response to the exposure temperature was investigated between 10 and 40 °C and was similar for all three strains of salmon lice (Fig. 20). Between the lowest exposure temperature of 10 °C and 25 °C, all copepods were observed to be active, then activity rapidly decreased between 30 °C and 35 °C (Fig. 20). Analysis of the dataset with GAMs showed that the best performing model included the smoothing term of temperature but not strain (p=0.345), indicating that louse strain is not likely to influence temperature tolerance (Fig. 20).
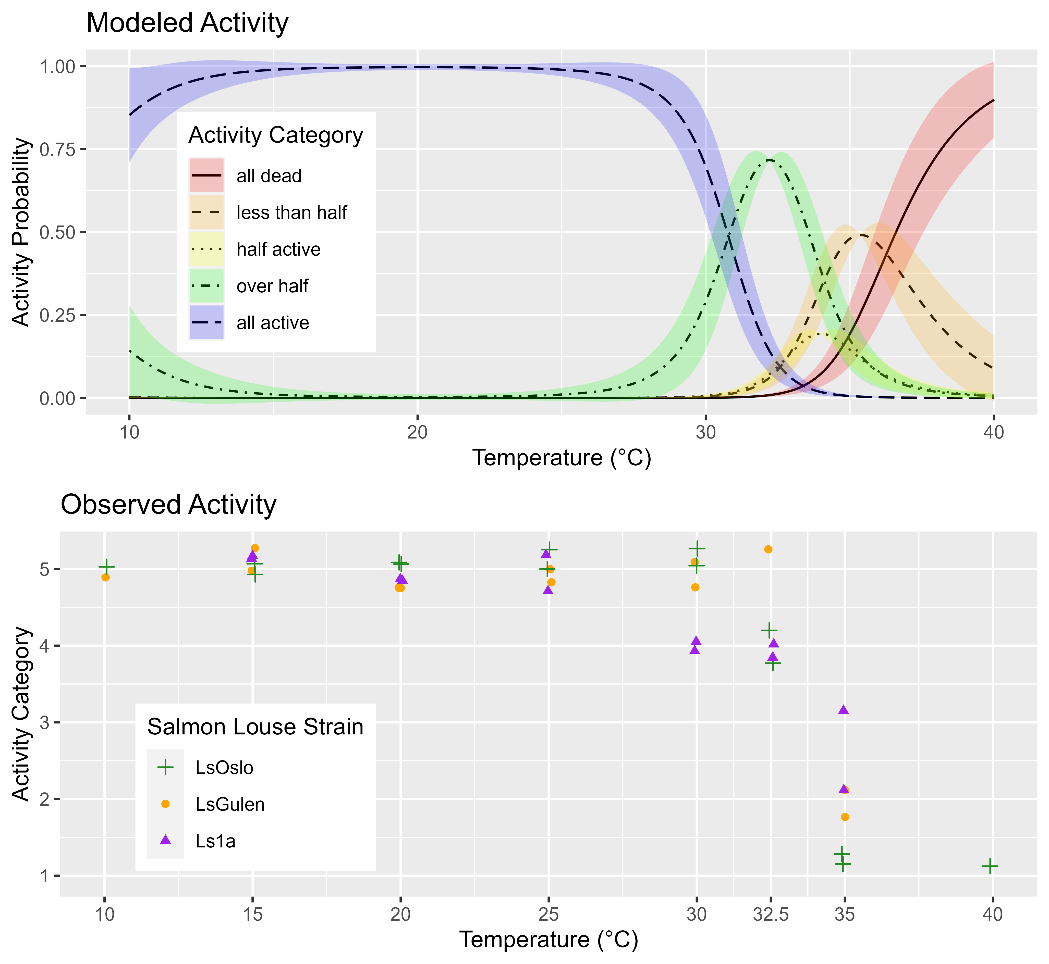
4.1.5 - Copepodid treatment and fish infection for inheritance determination
Copepodids from the selected Ls1a and LsOslo strains were cultured at 10 °C and full seawater salinity and synchronized to be 9.5 +/- 0.5 days post-hatch for the induced adaptation inheritance experiment. In these experiments the copepodids were treated at the highest temperature and lowest salinity that did not compromise the specific strains’ copepodid activity levels (determination of these values are described in sections 4.1.1 and 4.1.2). Cohorts from the LsOslofjord strain were treated at respectively 14 ppt and 25 °C, and cohorts from the Ls1a strain were treated at respectively 10 ppt and 30 °C using the method described in sections 4.1.1 and 4.1.2. Atlantic salmon (~500 g) were kept in individual fish tanks and infected with respectively P0 generation unexposed copepodids (as controls) and copepodids exposed to either warm - or low salinity water. This was done for three replicate fish for each treatment. The fish were groupwise infected in a communal infection bath (Fig. 21) supplied with app. 100 copepodids/fish. Each infection batch of copepodids was obtained from 2-3 pairs of egg-sacks depending on ne number of eggs pr. egg-sack.

4.1.6 - Acquired tolerance and epigenetic inheritance.
Synchronized copepodids from F1 (i.e. the offspring of the infections described in section 4.1.5) were monitored for phenotypic differences and sequenced to identify potential diagnostic 5-cM epimethylation markers. The F1 copepodids were challenged with high temperatures and low salinities as described in sections 4.1.1 and 4.1.2, but the temperature and salinity ranges were restricted to the approximate range where effects on the activity was seen (i.e. the lower temperatures and higher salinities described in Figs.18-20 were excluded). The phenotypic activities (Fig. 22) were determined as described in section 4.1.1.
Phenotypic differences in activity response to salinity and temperature level were examined with respect to parental exposure treatment and strain using GAMs (generalized additive models). The previous analysis indicated that for temperature the strain of the louse did not influence activity, and so GAMs were fitted to the entire temperature dataset to investigate the effect of parental exposure. The GAM which included just the smoothing term for temperature fit the data better (LRT, p=0.576) than one which included the smoothing term and a categorical treatment variable (control, parental exposure). The results indicate that in the experiment the previous generations’ exposure to a high temperature did not lead to greater temperature tolerance in the following generation (Fig. 22).
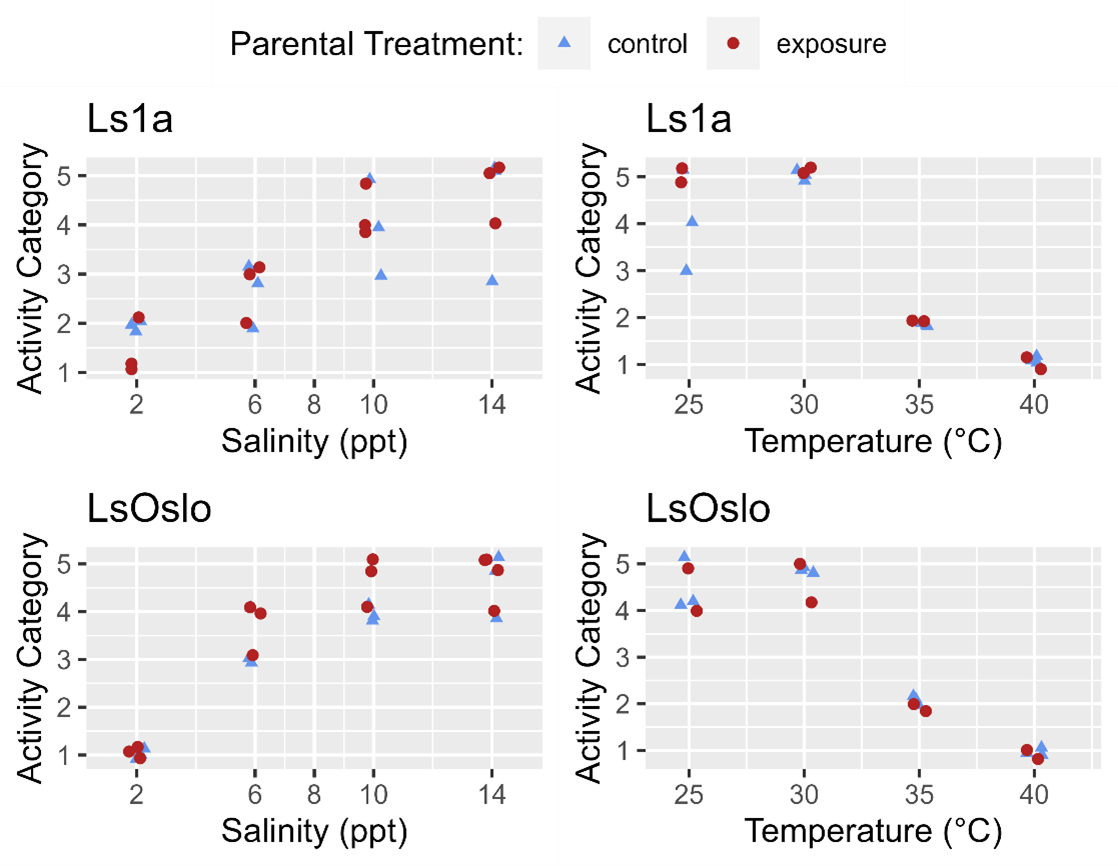
Since louse strains were shown to have different tolerances to salinity the salinity data was partitioned by strain to examine the effect of generation on activity. In the Ls1a dataset the GAM with only the smoothing term for salinity exposure fit the data better than one which included the smoothing term and a categorical treatment variable (LRT, p=0.649). Likewise, in the LsOslo dataset the likelihood ratio test indicated that the simpler model without the treatment variable and only the smooth term was the better fit (p = 0.080). Nonetheless, the GAM with both the smoothing term and treatment as a variable indicated that the copepodids from previously exposed parents were more active, and while it was not a significant difference at an α = 0.05 the p-value of 0.120 is suggestive of a difference (Fig. 23). In the GAM, the mean residuals of the parental exposed treatment group was also 8.3% greater than that of the control, indicating an increase in response variance.
4.1.7 - Inheritance of epigenetic markers induced by high temperatures and low salinities.
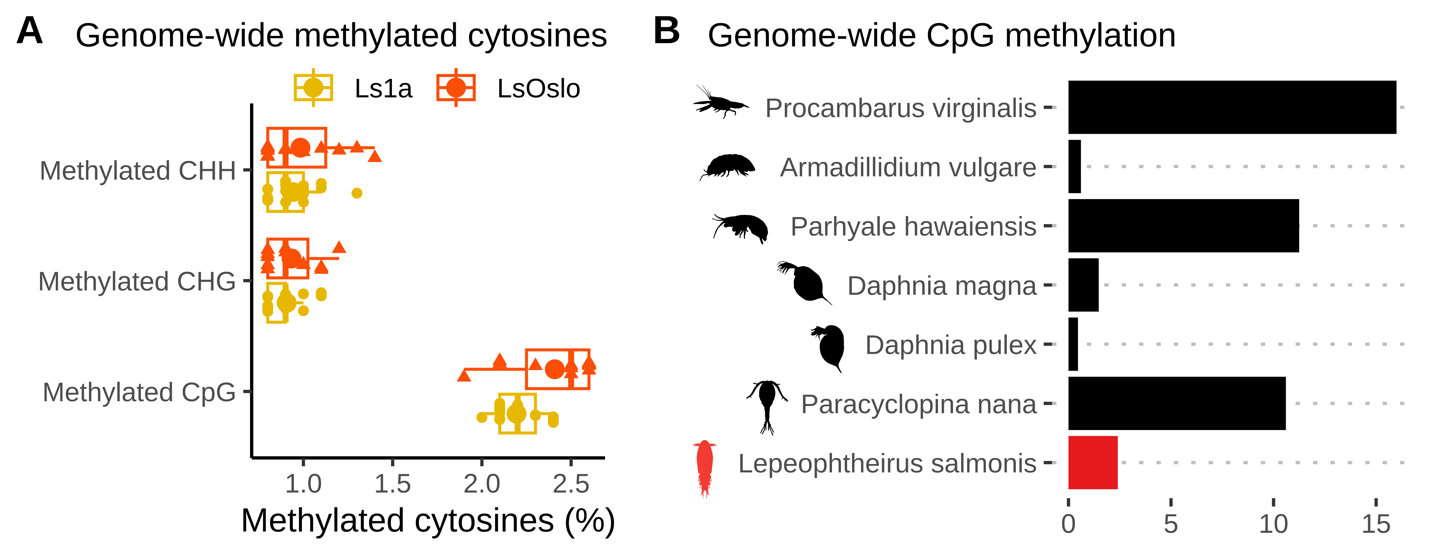
The global methylation levels in the obtained samples were monitored by antibody-based ELISA tests, and the results indicate a very low proportion of methylation (1-3%) in the copepodid samples compared to >50 in vertebrates. Unpublished results suggests that methylation is higher in adult lice (A. Borchel, University of Bergen, pers. com). Integrating these findings suggest that methylation increases during the salmon louse life cycle. The specific ontogenetic methylation dynamics is presently being investigated. Neither low nor high methylation levels would be contradictive to expectations since methylation in arthropods vary substantially (Lewis et al. 2020).
Samples of F1 copepodids were obtained for sequencing from each of six treatment groups (1: copepodid P0-treatment with low salinity, 2: copepodid P0-treatment with high temperature, and 3: control copepodids without P0-treatment from respectively Ls1a and LsOslofjord). To analyze DNA methylation patterns and identify potential DNA methylation markers, we sequenced 29 samples using reduced-representation bisulfite sequencing (RRBS), a fast and cost-effective method that targets a subset of CpG sites in the genome (i.e. sites with overabundance of Cytosines followed by Guanines, C’s and G’s, in the genetic code). Given the wide range of DNA-methylation patterns observed in invertebrates, we first verified that CpG sites were methylated in our samples. After aligning the processed sequence reads to the reference genome (LSalAtls2s; Skern-Mauritzen et al. 2021), we observed methylation rates of around 2 to 2.5% at the aligned CpG sites across all samples (Fig. 23A). To compare these rates with those in other crustacean species, we obtained genome-wide CpG methylation rates from previous studies ( Lewis et al. 2020, Lee et al. 2022). Our analysis revealed (Fig. 24) that the methylation levels in L . salmonis were higher than in some crustacean species (e.g. Daphnia magna and Daphnia pulex) but lower than in others (e.g. Paracyclopina nana and Procambarus virginalis). As previously noted, CpGs methylation levels in L. salmonis adults has been suggested to be higher than 2.5%, which suggests that the methylation levels change ontogentically in subsequent stages.
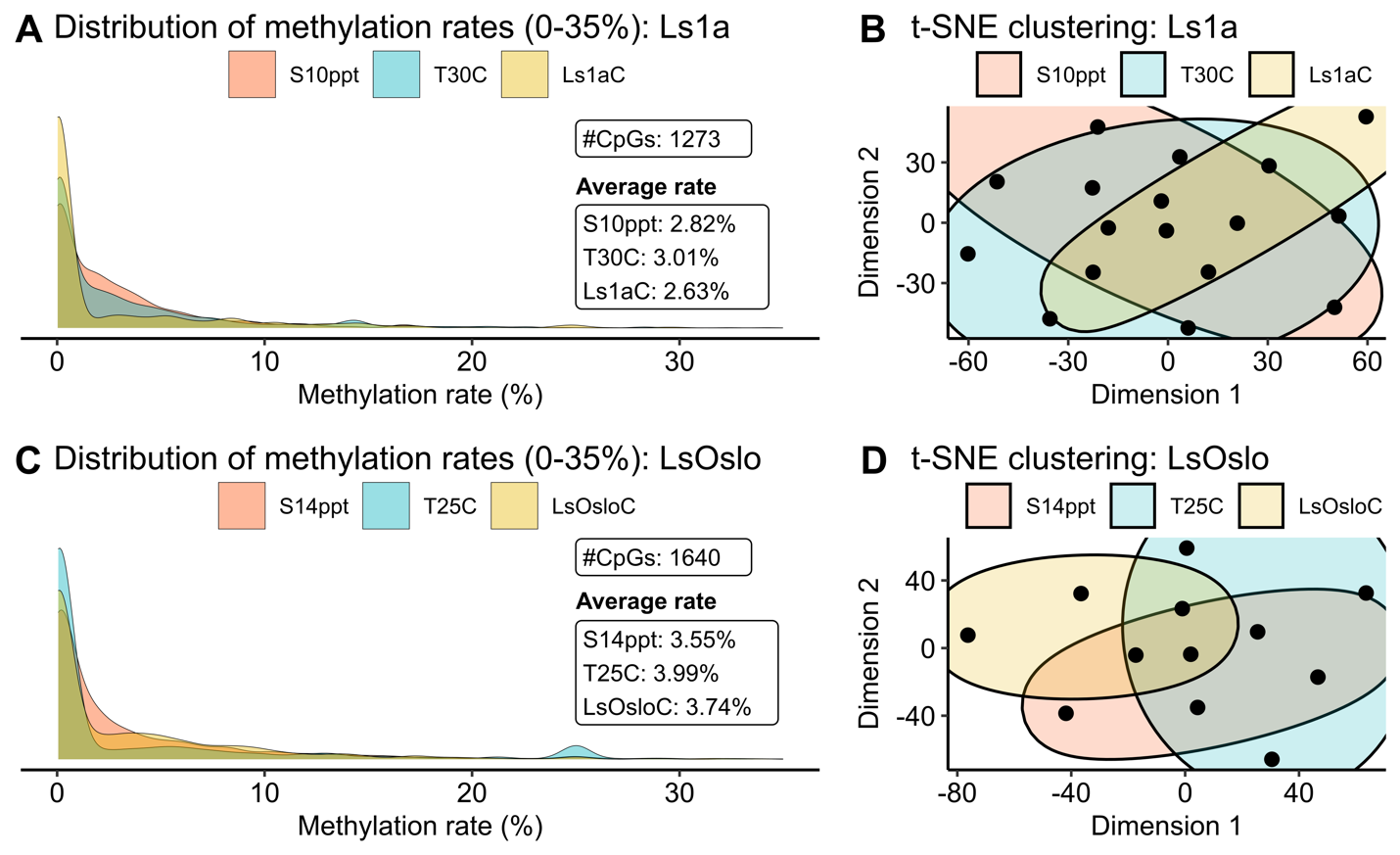
To facilitate a clear and concise comparison among treatments, we assigned names to the six groups as follows: S10ppt, T30C, and Ls1aC for Ls1a salinity, temperature, and control groups, and S14ppt, T25C, and LsOsloC for LsOslofjord salinity, temperature, and control groups. The methylation levels for Ls1a samples revealed that CpG sites were more frequently methylated in both the salinity - and temperature groups than in the control group (Fig. 25A). The group exposed to the temperature challenge exhibited the highest average methylation rates, followed by the salinity group and the control among the identified 1273 common CpG sites. However, the clustering analysis of methylation rates using these 1273 CpG sites did not reveal clear clusters, suggesting that the variation in methylation patterns between individual samples were larger than patterns induced by treatment (Fig. 25B). It should also be noted that even though the two treatment groups displayed higher average CpG methylation rates than the control group, the hyper-methylated CpG sites were not necessarily conserved within the group.
Similar trends in the density and clustering were observed when LsOslofjord samples were analyzed (Fig. 25C,D) although, two significant differences were found. Firstly, the salinity treatment showed the lowest methylation levels, in contrast to Ls1a where the control group showed the lowest methylation levels. Secondly, the average methylation levels for all LsOslofjord groups were higher than the levels found in the Ls1a groups. The difference between treatment response suggest that strains may show different methylation responses to challenges. The difference in overall methylation levels between the strains may suggest strain specific differences in methylation levels, or the difference may suggest that LsOslofjord has a slightly faster growth rate and that an ontogenetic methylation increase has progressed further than in Ls1a.
4.1.8 - Resilience markers in field samples
Analysis of field samples depended on successful identification of consistent treatment-specific epimethylation markers. We attempted to identify DNA methylation markers through differential methylation analysis to identify CpG sites that were differentially methylated with statical significance. These CpG sites were then used as candidate sites for markers. We utilized a logistic regression combined with lasso regularization to select a set of markers for each treatment. The logistic regression revealed 27 CpG sites as four sets of DNA methylation marker candidates without any overlaps (Fig. 26). Each set of candidate markers was able to perfectly distinguish the treatment samples from the others within a strain. For example, the first set of markers (Fig. 26A) effectively distinguished samples in the Ls1a salinity group from those in the other two Ls1a groups. In the attempt to identify DNA methylation markers, we conducted differential methylation analysis to obtain CpG sites that were differentially methylated with statically significance. These were then used as candidate sites for markers. We utilized a logistic regression combined with lasso regularization to select a set of markers for each treatment. The logistic regression revealed 27 CpG sites as four sets of DNA methylation marker candidates without any overlaps (Fig. 26). Each set of candidate markers was able to perfectly distinguish the treatment samples from the others within a strain. For example, the first set of markers (Fig. 26A) effectively distinguished samples in the Ls1a salinity group from those in the other two groups.
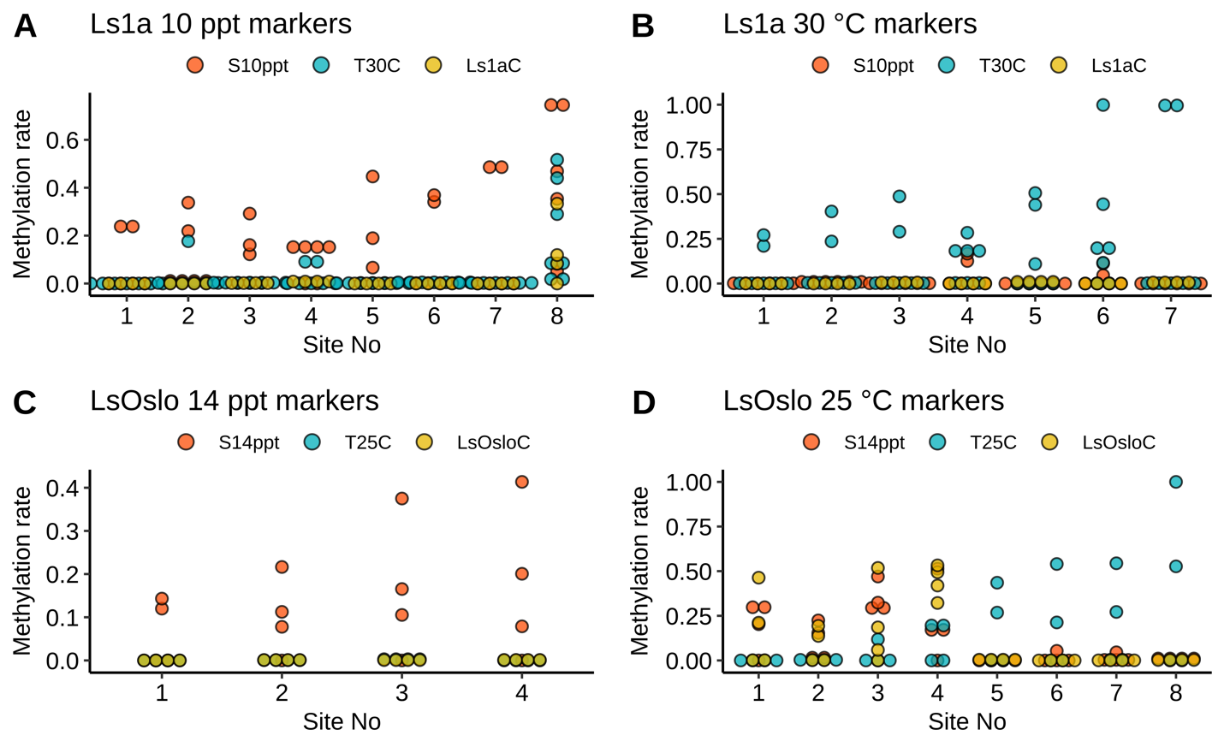
To be useful, the identified markers must be applicable across strains - and in uncharacterized wild lice. To validate the robustness and effectiveness of the markers, we compared results from the treatment between two strains. The validation showed that only one out of four sets of markers from the LsOslo low salinity group were also found in the Ls1a group – and these markers were able to correctly predict the true treatment status only in a fraction of these samples. For example, one marker from the LsOslofjord strain correctly identified only four out of six Ls1a salinity samples. This shows that the identified markers were not robust and suggested that considerable further investigation is required to identify robust markers that would work across different strains and in wild samples. Based on these conclusions attempts to investigate identified epigenetic resilience markers in field samples were not carried out.
5 - Key findings
Key findings in brief (English)
-
Single freshwater bath treatments and freshwater baths combined with other treatments had the greatest delousing efficacy, while hyposaline (ion-modified) water had a strong delousing effect compared to seawater. Results also suggested that handling alone is responsible for a significant loss of lice.
-
Welfare status of fish were generally similar across single and combination treatments, however combination treatments that included a 34°C thermal bath caused poorer welfare outcomes. The inclusion of procedural controls showed that the handling associated with treatments, rather than the treatments themselves, was the major contributor to poor welfare outcomes.
-
Decisions on delousing treatments applied at commercial sites were largely balanced between welfare needs and production goals, where riskier operations were used when fish were perceived to be in better health condition. Mortality was associated with any handling event, and higher for thermal and mechanical treatments.
-
Evidence of differences at the epigenetic level was detected in copepodids exposed to low salinity water, however diagnostic markers for exposure and resilience for this larval stage were not identified. Such potential markers should be investigated in pre-adult or adult stages.
Key findings in brief (Norwegian)
-
Ferskvannsbadbehandling, alene eller kombinert med andre behandlinger, hadde størst avlusningseffekt. Hyposalin (ionemodifisert) vann hadde større avlusningseffekt enn normalt sjøvann. Resultatene tyder også på at håndtering alene førte til at lus falt av.
-
Konsekvensene for fiskens velferdsstatus var generelt lik mellom enkelt- og kombinasjonsbehandlingene, men kombinasjonsbehandlingene som inkluderte et 34°C termisk bad førte til mer skade. Prosedyrekontrollene viste at håndteringen knyttet til behandlingene, snarere enn selve behandlingene, var den største årsaken til at fisk ble skadet.
-
Beslutninger om avlusingsbehandling brukt på kommersielle oppdrettsanlegg var i stor grad en avveining mellom fiskestatus og produksjonsmål, der mer risikofylte behandlinger ble foretrukket når fisken ble ansett å ha robust helse og velferd. Alle behandlingsformene medførte risiko for økt dødelighet, men risikoen var høyest for termiske og mekaniske behandlinger.
-
Forskjeller på epigenetisk nivå ble påvist i kopepoditter eksponert for vann med lav saltholdighet, men diagnostiske markører for eksponering og motstandsdyktighet for dette larvestadiet ble ikke identifisert. Slike potensielle markører bør undersøkes for pre-adulte og adulte stadier.
The overarching objective of the project was to compare efficacy of single and combined treatments to understand under what conditions they function best, and how they impact salmon welfare. Secondary objectives were to further investigate this at commercial scale, and to explore epigenetic freshwater and thermal resilience in lice.
Results from the three rounds of WP1 experiments (Section 2) indicated that single freshwater bath treatments and freshwater baths combined with other treatments had the greatest delousing efficacy. An add-on experiment showed that hyposaline (ion-modified water) had a strong delousing effect compared to seawater. In all treatment groups progression through the experiments was associated with a decline in lice load suggesting that handling alone is responsible for a significant loss of lice. Across experiments, the combined treatments with a 4-hour freshwater bath had slight, if any, additional delousing effect over the single 20-hour freshwater bath treatment. Thus, the marginal increase in efficacy should be considered in balance with the additional welfare impact and possibility of resilience building. The data suggested the maximum combination of treatments with the 34°C thermal bath contributes to greater welfare severity. Nevertheless, the welfare assessments show that while all treatment groups and experimental conditions are associated with worse welfare outcomes, no single treatment or combination had a significantly worse outcome than the procedural controls. Overall, the findings support the hypothesis that the handling associated with delousing treatments is a major contributor to poor welfare outcomes in the treated fish.
Delousing efficacy and welfare impacts at commercial scale were initially investigated through site visits for WP2. Those visits were informative and led directly to the add-on hyposaline trial, but further site visits were deemed too logistically challenging for the project goals. Rather than have the project team coordinate with commercial partners and do the sampling on site, we gained direct access to industry data through a complimentary project (Section 3). The investigation revealed that farmers’ decisions balance welfare needs with production goals, but their choices are constrained by regulations. Typically, farmers choose to apply riskier handing operations on fish which are perceived to be stronger while reserving gentler operations for those that are perceived to be weaker. Analysis of the dataset was complicated by those confound factors but nonetheless showed that mortality increases after all handling events and delousing operations. The greatest mortality increases were associated with well boat operations including thermal and mechanical treatments. Those non-medicinal treatments had higher delousing efficacy of mobile stages compared to bath treatments but treatment effects on attached stages could not be determined due to unreliable count data. Moving operations done under low sea temperatures (4-6°C) were associated with higher rates of mortality than those done under more moderate temperatures (9°C).
In WP3 (Section 4) the temperature and low salinity tolerance levels, and the ability for these tolerance levels to shift upon exposure, were investigated. Three strains were investigated and while their temperature tolerance levels were similar, significant differences in salinity tolerance levels between strains were found. When comparing copepodids (F1) from exposed and unexposed parents (P0) no significant differences were found. A more detailed investigation at the epigenetic level (specifically targeting 5-mC DNA epimethylation) revealed significant differences between groups. These differences were not sufficiently consistent to allow development of diagnostic assays. However, considering that differences were found and that treatments were performed only at the copepodid stage , the potential for salinity tolerance markers to be found in future studies should not be disregarded.
6 - References
Bang Jensen B, Qviller L, Toft N (2020) Spatio-temporal variations in mortality during the seawater production phase of Atlantic salmon (Salmo salar) in Norway. J Fish Dis 43:445-457
Coates A, Phillips BL, Oppedal F, Bui S, Overton K, Dempster T (2020) Parasites under pressure: salmon lice have the capacity to adapt to depth-based preventions in aquaculture. Int J Parasitol 50(10-11):865-872
Gismervik K, Nielsen KV, Lind MB, Vijugrein H (2017) Mekanisk avulsing med FLS-avlusersystem = dokumentasjon av fiskevelferd og effekt mot lus. Norwegian Veterinary Institute’s Report Series: 6-2017.
Grefsrud ES, Bjørn PA, Grøsvik BE, Hansen PK, Husa V, Karlsen Ø, Kvamme BO, Samuelsen O, Sandlund N, Solberg MS, Stien LH (2022) Risikorapport norsk fiskeoppdrett 2022 – kunnskapsstatus — Effekter på miljø og dyrevelferd i norsk fiskeoppdrett. Rapport fra Havforskningen, 2022-13.
Hamre LA, Bui S, Oppedal F, Skern-Mauritzen R, Dalvin S (2019) Development of the salmon louse Lepeophtheirus salmonis parasitic stages in temperatures ranging from 3 to 24 degrees C. Aquac Environ Interact 11:429-443
Jablonka E (2017) The evolutionary implications of epigenetic inheritance. Interface Focus 7(5)
Jeremias G, Barbosa J, Marques SM, De Schamphelaere KAC, Van Nieuwerburgh F, Deforce D, Goncalves FJM, Pereira JL, Asselman J (2018) Transgenerational Inheritance of DNA Hypomethylation in Daphnia magna in Response to Salinity Stress. Environ Sci Technol 52(17):10114-10123
Johansen IB, Höglund E, Øverli Ø (2020) “Individual Variations and Coping Style." in The Welfare of Fish, pp. 283-301. Springer
Lewis SH, Ross L, Bain SA, Pahita E, Smith SA, Cordaux R, et al. (2020) Widespread conservation and lineage-specific diversification of genome-wide DNA methylation patterns across arthropods. PLoS Genet 16(6): e1008864. https://doi.org/10.1371/journal.pgen.1008864
Lee YH, Kim MS, Wang M. et al. (2022) Epigenetic plasticity enables copepods to cope with ocean acidification. Nat. Clim. Chang. 12, 918–927. https://doi.org/10.1038/s41558-022-01477-4
Ljungfeldt LER, Quintela M, Besnier F, Nilsen F, Glover KA (2017) A pedigree-based experiment reveals variation in salinity and thermal tolerance in the salmon louse, Lepeophtheirus salmonis. Evolutionary Applications 10(10):1007-1019
Macaulay G, Barrett LT, Dempster T (2022) Recognising trade-offs between welfare and environmental outcomes in aquaculture will enable good decisions. Aquaculture Environment Interactions, 14, 219-227.
Moore G (2021) Thermal delousing allowed to continue in Norway. In: Fish Farming Expert. Gustav-Erik Blaalid, Bergen Norway, https://www.fishfarmingexpert.com/norwegian-food-safety-authority-salmon-farming-sea-lice/thermal-delousing-allowed-to-continue-in-norway/1383096
Nilsson J, Moltumyr L, Madaro A, Kristiansen TS, Gåsnes SK, Mejdell CM, Gismervik K, Stien LH. (2019) Sudden exposure to warm water causes instant behavioural responses indicative of nociception or pain in Atlantic salmon. Veterinary and Animal Science 8:100076.
Oliveira VHS, Dean KR, Qviller L, Kirkeby C, Bang Jensen B (2021) Factors associated with baseline mortality in Norwegian Atlantic salmon farming. Scientific reports, 11: 14702
Overton K, Dempster T, Oppedal F, Kristiansen TS, Gismervik K, Stien LH (2019) Salmon lice treatments and salmon mortality in Norwegian aquaculture: a review. Reviews in Aquaculture. 11:1398-1417.
Persson D, Nødtvedt A, Aunsmo A, Stormoen M (2022) Analysing mortality patterns in salmon farming using daily cage registrations. Journal of Fish Diseases, 45(2), 335-347.
Powell, MD, Reynolds P, Kristensen T (2015) Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquaculture, 448, 18-28.
Rechavi O, Minevich G, Hobert O (2011) Transgenerational Inheritance of an Acquired Small RNA-Based Antiviral Response in C. elegans. Cell 147(6):1248-1256
Skern-Mauritzen R, Malde K, Eichner C, Dondrup M, Furmanek T, Besnier F, Komisarczuk AZ, Nuhn M, Dalvin S, Edvardsen RB et al: The salmon louse genome: Copepod features and parasitic adaptations. Genomics 2021, 113(5):3666-3680.
Stien LH, Thompson CRS, Fjelldal PG, Oppedal F, Kristiansen T, Sæther PA, Bølgen PM, Martinsen L (2023) Production, Fasting and Delousing of Triploid and Diploid Salmon in Northern Norway: Report for the 2020 Generation. Rapport fra Havforskningen, 2023-20
Thompson C, Madaro A, Stien LH, Nilsson J, Øverli Ø, Korzan W, Bui S (2023a) Comparison of non-medicinal delousing strategies for parasite (Lepeophtheirus salmonis) removal efficacy and welfare impact on Atlantic salmon (Salmo salar) hosts. In press in Aquaculture International
Thompson C, Madaro A, Oppedal F, Stien LH, Bui S (2023b) Delousing efficacy and physiological impacts on salmon of freshwater and hyposaline bath treatments. Rapport fra Havforskningen, 2023-11.
Walde CS, Jensen BB, Pettersen JM, Stormoen M (2021) Estimating cage‐level mortality distributions following different delousing treatments of Atlantic salmon (Salmo salar) in Norway. Journal of Fish Diseases.
Zuur AF, Ieno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7:636-645