Havforskningsinstituttet har undersøkt prevalensen av infeksiøs lakseanemivirus (ILAV), salmonid alfavirus (SAV, PD-virus), piscine orthoreovirus 1 (PRV-1), piscine myokardittvirus (PMCV) og bakteriell nyresyke (BKD) infeksjoner i vill atlantisk laks postsmolt og sjøøret fanget i 2023 i tre akvakulturproduksjonsområder (PO2, 3 og 6). Fisken ble samlet inn som en del av det nasjonale overvåkingsprogrammet for lakselus. Postsmolten ble tatt i ytre deler av Boknafjord (N = 50) og Hardangerfjord (N = 50) ved tråling i perioden mai-juni. Sjøøretten ble fanget ved ruse og garn i Hitra område i juni. Lave konsentrasjoner av SAV-RNA ble påvist i to postsmolt fra Boknafjorden og én postsmolt fra Hardangerfjorden. ILAV ble påvist i én fisk fra Boknafjorden, én postsmolt fra Hardangerfjorden og én sjøørret fra Hitra. PRV1 ble påvist i 6 postsmolt, 5 fra Boknafjorden og en fra Hardangerfjorden. Lave konsentrasjoner av PMCV ble påvist i 9 fisk. Den PMCV-positive postsmolten ble samlet inn fra begge fjordene. Det var én PMCV-positiv sjøørret fra Hitra. Viruspositiv fisk hadde høye Ct-verdier (34-40) og disse verdiene representerer sannsynligvis svake eller falske positive resultater. Renibacterium salmoninarum (BKD agens) ble ikke påvist i noen av postsmolt eller sjøørret. Resultatene i den rapporten viste at postsmolt og sjøørret fra fjorder hadde svært lav forekomst av patogensmitte som er vanlig i norsk oppdrett. Disse funnene utfyller og bekrefter de tidligere rapporterte dataene våre og kan tyde på at prevalensen av patogeninfeksjoner i villaks postsmolt og sjøørret ikke påvirkes signifikant av forekomsten av disse infeksjonene i fiskeoppdrett.
Annual report on health monitoring of wild anadromous salmonids in Norway 2023
— Screening of Atlantic salmon postsmolts from Boknafjorden and Hardangerfjorden and sea trout from Hitra for pathogen infections
Rapportserie:
Rapport fra havforskningen 2024-13
ISSN: 1893-4536
Overvåkingsgruppens rapporter
Publisert: 08.04.2024
Oppdatert: 15.04.2024
Prosjektnr: 15697-01
Oppdragsgiver(e): Norwegian Food Safety Authority
Forskningsgruppe(r):
Smittespredning og sykdom
Tema:
Laks ,
Ørret/aure
Program:
Miljøeffekter av akvakultur
Forskningsgruppeleder(e):
Sussie Dalvin (Smittespredning og sykdom)
Godkjent av:
Forskningsdirektør(er):
Geir Lasse Taranger
Programleder(e):
Mari Skuggedal Myksvoll
English summary
Sammendrag
The Institute of Marine Research has investigated the prevalence of infectious salmon anemia virus (ISAV), salmonid alphavirus (SAV, PD virus), piscine orthoreovirus 1 (PRV-1), piscine myocarditis virus (PMCV) and bacterial kidney disease (BKD) infections in wild postsmolt Atlantic salmon and sea trout caught in 2023 in three aquaculture production areas (PO2, 3 and 6). The fish were collected as part of the national monitoring program for salmon lice. The postsmolts were taken in outer parts of Boknafjord (N = 50) and Hardangerfjord (N = 50) by trawling in the period May-June. The sea trout was caught using gillnet and fish trap in Hitra area in June. Low concentrations of SAV-RNA were detected in two post smolts from the Boknafjord and one post smolt from the Hardangerfjord. ISAV was detected in one fish from Boknafjorden, one post smolt from Hardangerfjorden and one sea trout from Hitra. PRV1 was detected in 6 post smolts, 5 from Boknafjorden and one from Hardangerfjorden. Low concentrations of PMCV were detected in 9 fish. The PMCV-positive postsmolts were collected from both fjords. There was also one PMCV-positive sea trout from Hitra. Virus-positive fish had high Ct-values (34-40) and these values represent probably weak or false positive results. Renibacterium salmoninarum (causative agent of BKD) was not detected in any postsmolt or sea trout. The results in the current report showed that postsmolt and sea trout from fjords had a very low occurance of pathogen infections, which is common in Norwegian aquaculture. These findings complement and confirm our previously reported data and may indicate that the prevalence of pathogen infections in wild salmon post-smolt and sea trout is not significantly affected by the occurrence of these infections in fish farming.
1 - Introduction
Infectious diseases in Atlantic salmon farming in Norway is a serious problem which has an impact on welfare of infected salmon and often leads to substantial economic losses (Table 1) [1]. The most common viral diseases in salmon farming are pancreas disease (PD), caused by salmonid alphavirus (SAV), infectious salmon anaemia (ISA), caused by ISA virus (ISAV), heart and skeletal muscle inflammation (HSMI), caused by piscine orthoreovirus 1 (PRV1) and cardiomyopathy syndrome (CMS), caused by piscine myocarditis virus (PMCV).
PD is one of the major diseases in fish farming and much of the fish in endemic areas are believed to become infected with SAV through a production cycle. PD outbreaks have been dominated by SAV2 in central Norway and SAV3 in western Norway [1,2]. ISA is a serious disease that has led to severe epizootics in salmon aquaculture in Faroe Islands, Norway and Chile, with enormous economic consequences for industry. Since the 1993, there have been relatively few ISA outbreaks in Norway. However, an increase in the number of ISA outbreaks in recent years along the entire Norwegian coast indicates a growing problem in salmon farming. There are two variants of the virus, one that causes disease (virulent, HPR-del) and one that does not cause disease (avirulent, HPR-0). There is growing evidence that virulent ISAV arises from avirulent ISAV, but what triggers this is not yet known [3]. Heart and skeletal muscle inflammation (HSMI) is the most abundant viral disease in salmon farming. The disease is an increasing problem in fish farming in Norway with 79–188 annual registered cases of HSMI in recent years [1,4]. High PRV-1 viral loads are found in fish developing HSMI but may also occur in healthy fish. Cardiomyopathy syndrome (CMS) is a growing problem in Norwegian salmon farming with 82 to 155 annual outbreaks in the last 5 years [1,5].
Bacterial kidney disease (BKD) is a serious chronic systemic infection in salmonids that is caused by and Renibacterium salmoninarum. It is a notifiable disease and has been sporadically reported in farmed and wild salmonids in Norway in the last 20 years. However, 12 BKD-outbreaks were reported in production areas 4 (N=3) and 6 (N=9) in 2023[1]. The sudden increase of the outbreak numbers is alarming and needs further attention from the fish farming industry as well as the management authorities.
|
2019 |
2020 |
2021 |
2022 |
2023 |
|
|---|---|---|---|---|---|
|
PD |
152 |
158 |
100 |
98 |
58 |
|
ISA |
10 |
23 |
25 |
15 |
18 |
|
HSMI |
79 |
161 |
188 |
147 |
184 |
|
CMS |
82 |
154 |
155 |
131 |
129 |
|
Total |
323 |
496 |
468 |
391 |
389 |
Pathogen exchange between farmed and wild salmon occurs, e.g. salmon lice. Hence, disease outbreaks in salmon farms may lead to increased infection pressure on wild fish populations. There is an increasing public concern that this may have negative impact on wild salmonids in Norway. However, although the amount of the data and knowledge on the prevalence of virus infections in wild salmonid populations in Norway is increasing [6,7], it is still limited. Furthermore, it is difficult to quantify disease incidence and its impact on wild fish since sick individuals may be less catchable or may disappear in nature unnoticed (e.g. due to predation). Therefore, it is challenging to evaluate the impact of pathogens on individuals as well as stocks, since we normally are only able to collect infected but non-diseased fish such as individuals that has recently acquired or has survived an infection (carriers). To increase our knowledge, long term surveillance programmes creating a timeseries with sufficient data are necessary.
The effect of fish farming on the infection status of wild salmon stocks may be evaluated by comparing pathogen prevalence in wild fish populations originating from areas with different fish farming intensities and disease outbreak profiles.
Wild salmon may be infected by pathogens prevalent in salmon farming; in rivers as fry or parr by virus-infected farmed escapees or spawning wild salmon, or from salmon farms in the fjord when migrating as smolts or returning as adults. Therefore, infection status in migrating smolts may represent a direct indicator of infection pressure from salmon farming during their migration routes. However, the study of pathogen infection during all the life stages of salmon is necessary to assess the overall impact of diseases in fish farming on the wild salmon stocks.
Since 2012, the Institute of Marine Research (IMR) has been commissioned by the Norwegian Food Safety Authority (NFSA) to carry out an annual health monitoring of wild anadromous salmonids in Norway. The current monitoring activities are financed by both NFSA and the Norwegian Ministry of Trade, Industry and Fisheries (NFD). The activities lie within a prioritized research area at IMR which addresses the environmental impact of disease transmission from Norwegian fish farming to wild fish. The surveillance activities aim to evaluate the virus transmission from farmed fish to wild salmonids by monitoring and identifying changes in the prevalence of selected pathogens in wild salmonids as a result of fish farming activities. In addition, the surveillance aims to increase the knowledgebase about pathogens in wild salmonids in general, as well as establish a biobank that can be used when new disease challenges arise. Furthermore, the surveillance consolidates with the other activities in the larger strategic research effort on diseases and disease transmission in wild fish.
Part of the research activities in the surveillance program aims to generate data about:
- Pathogen prevalence in fry, parr, postsmolt and returning adult salmon.
- Prevalence of Pathogens in sea trout.
- Prevalence of infections in escaped farmed salmonids.
- Genotypes and characteristics of detected pathogens.
The virus screening is based on selected materials obtained through monitoring of pathogen infections in wild salmonids project and other associated projects at IMR, such as:
- National salmon lice monitoring program (NALO).
- National escaped salmon monitoring program.
- Etne research station (fish trap).
The current monitoring program aims to investigate the occurrence of pathogen infections in wild salmonids captured from different Norwegian coastal areas with different farming intensities and disease outbreak frequencies. Each year selected sets of fish are analysed in order to complement or complete our data and time series. Part of the results from pathogen screening are used in an annual health monitoring of wild anadromous salmonids in Norway commissioned by NFSA. The generated knowledge from the program contributes to the institute's main goal/strategy in providing advice and further development of sustainable management of aquaculture and is utilized in the IMR's annual risk assessment of Norwegian fish farming.
For 2023, the OK program investigated the occurrence of SAV, ISAV, PRV1 and PMCV infections in migrating postsmolt from the Boknafjorden and Hardangerfjorden and sea trout from Nordfjorden. Sea trout will be also tested for Renibacterium salmoninarum (the causal agent of BKD). The three collection sites are located in 3 different salmon production areas (PO) that have different farming intensities and disease profiles and were therefore selected based on an assessment of the risk of infection by these pathogens [6,7]. Fish from each site were analysed for virus infections using real-time RT-PCR.
2 - Aim
The aim of the current study was to investigate the occurrence of SAV, ISAV, PRV1, and PMCV infections in migrating wild Atlantic salmon postsmolts captured in 2023 in two fjord systems located in two aquaculture production areas (PO2 and PO3) with differing risk for virus infection of wild fish. Additionally, the occurrence of SAV, ISAV, PRV1, PMCV and Renibacterium salmoninarum (BKD) infections were tested in sea trout from PO6 (Hitra).
3 - Materials and methods
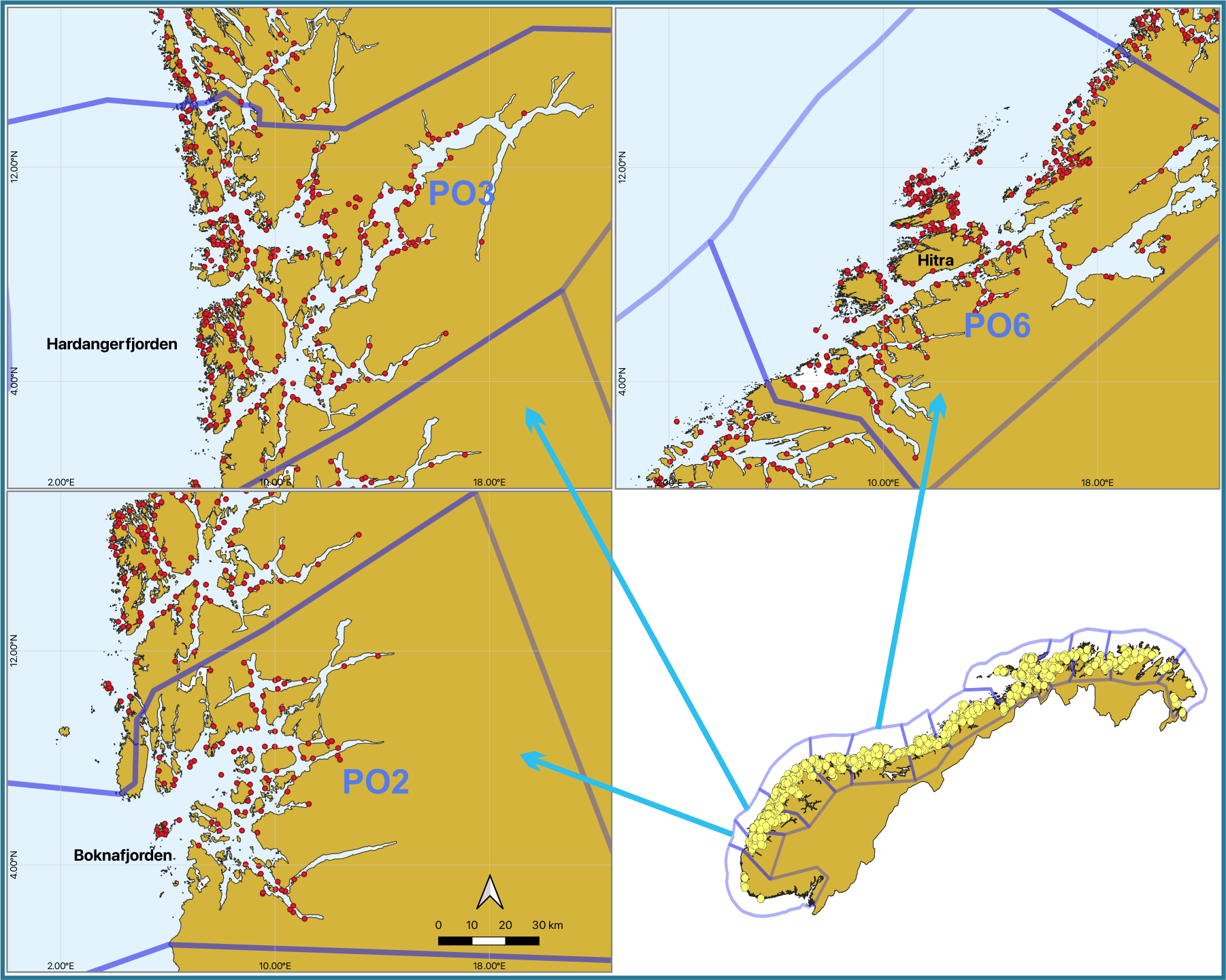
To provide data about the prevalence of different viruses in different salmon life stages and different geographical regions, we randomly selected fish caught by trawl as part of the national salmon lice monitoring program (NALO) [8]. The salmon postsmolts were caught in the outer parts of Boknafjorden and Hardangerfjorden during the period May-June 2023 (Fig. 1 and Table 2). The trawl data are available at nmdc.no. The sea trout were caught Hitra area using gillnet and fish trap in June 2023.
To provide data about the prevalence of different viruses in different salmon life stages and different geographical regions, we randomly selected fish caught by trawl as part of the national salmon lice monitoring program (NALO) [8]. The salmon postsmolts were caught in the outer parts of Boknafjorden and Hardangerfjorden during the period May-June 2023 (Fig. 1 and Table 2). The trawl data are available at nmdc.no. The sea trout were caught Hitra area using gillnet and fish trap in June 2023.
Salmon lice infestation (count), weight and length of all postsmolts and seat rout were recorded, and the fish were then frozen (-20 oC) as soon as possible. At autopsy, tissues from the gills, head kidney and heart were taken from the fish while still frozen and stored at -80 oC. Samples for analysis were sent on dry ice to an accredited commercial laboratory for RNA extraction and pathogen testing (Pharmaq Analytiq AS; https://www.pharmaq.com/en/analytiq). All fish were tested for SAV, ISAV, PRV1, PMCV and Renibacterium salmoninarum (Table 2) by real-time RT-PCR assays (for detection of pathogen RNA). A total of 690 analyses were performed on 138 fish and included in the current report.
4 - Results
Low concentrations of SAV-RNA were detected in two postsmolts from Boknafjorden and one postsmolt from Hardangerfjorden (Table 2). ISAV was detected in one fish from Boknafjorden, one postsmolt from Hardangerfjorden and one sea trout from Hitra. PRV1 was detected in 6 postsmolts, 5 from Boknafjorden and one from Hardangerfjorden. Low concentrations of PMCV were detected in 9 fish. The PMCV-positive postsmolts were collected from both fjords. There was one PMCV-positive sea trout from Hitra. Virus-positive fish had high Ct-values (34-40) and these values are likely to represent weak or false positive results. Renibacterium salmoninarum was not detected in any of postsmolts or sea trout.
|
Collection Site (production area) |
N. |
SAV |
ISAV |
PRV1 |
PMCV |
R. Sal.* |
|---|---|---|---|---|---|---|
|
Postsmolt Boknafjorden (PO2) |
50 |
2 (4%) |
1 (2%) |
5 (10%) |
7 (14%) |
0 (0%) |
|
Postsmolt Hardangerfjorden (PO3) |
50 |
1 (2%) |
1 (2%) |
1 (2%) |
2 (4%) |
0 (0%) |
|
Sea trout Hitra (PO6) |
38 |
0 (0%) |
1 (3%) |
0 (0%) |
1 (3%) |
0 (0%) |
|
Total |
138 |
3 (2%) |
3 (2%) |
6 (4%) |
10 (7%) |
0 (0%) |
|
PCR Ct-values |
|
38-40 |
35-36 |
34-39 |
36-40 |
- |
* Renibacterium salmoninarum
5 - Discussion and Conclusion
Low concentrations of SAV were detected in two migrating postsmolt from production area PO2 and one postsmolt from PO3. During 2023, the numbers PD-outbreaks were none, 11 and 21 in productions areas PO2, PO3 and PO6 respectively. Therefore, it is likely that migrating postsmolts from PO3 PO6 were exposed to SAV released from the fish farms. However, the very low concentrations (Ct-values: 38-40) was just below the detection limit of the PCR assay (cut-off 37) and makes it difficult to conclude if the results indicating weak or false positive. Our earlier reports showed also very low prevalence of SAV in returning adult salmon, postsmolt at Norwegian sea or juveniles from Norwegian rivers [6,7,10-15]. The low prevalence of SAV infections in the tested migrating smolt is consistent with previous findings in wild salmonids [9-14] which suggest that the prevalence of virus infections in wild salmonids is not significantly affected by diseases in fish farming.
ISAV was detected in three (2%) fish. There was 4, 8 and 2 ISA-outbreaks in the PO2, PO3 and PO6 respectively, during 2023 [1,7]. Both ISAV-positive postsmolts had a Ct-value of 36, whilst the ISAV-positive sea trout had a Ct-value of 35. The very low virus concentrations (high Ct-values) did not allow to determine the virulence (HPR-0 or HPR-del) of the virus.
PRV1 infection is abundant in fish farming in production areas PO2-13. We detected the virus in 6 (4%) postsmolts from PO2 and PO3 (Table 2). All the PRV1-positive fish had high Ct-values (34-39). PRV1 has been detected in wild salmon and sea trout by real-time RT-PCR [9-15]. Reports has shown that there was no regional pattern in virus genotypes isolated from wild and farmed salmon, suggesting prolonged and extensive spread due to aquaculture activities (fish transport) and frequent exchange of the virus types between farmed and wild fish [10,16]. However, little is known about the mechanism of transmission of the virus.
PMCV was detected at low concentrations (ct-values 36-40) in 10 (7%) of the tested fish. The ct-values of the RT-PCR is likely to indicate false positive or very low virus concentrations. Previous reports have shown low prevalence of PMCV infections in wild salmon and that infection was not associated with fish farming activities [6,13,17].
There was no BKD-outbreaks in PO2 and PO3 during 2023. On the other hand, 9 BKD-outbreaks reported in PO6 in 2023. All salmonids (including sea trout) are susceptible to infection by Renibacterium salmoninarum which is the causative agent of BKD. However, Renibacterium salmoninarum was not detected in any of the tested fish irrespective of collection area or the number of BKD outbreaks. Furthermore, there was 3 BKD-outbreaks in P4 in 2023. Testing of 100 migrating potsmolt collected in 2023 from PO4 (Nordfjorden) did not reveal any Renibacterium salmoninarum infection in the fish (data are not shown). Our results from screening suggest that despite the increased number of BKD-outbreaks in PO4 and PO6, wild salmonids from the areas were not infected with the causative agent (Renibacterium salmoninarum). However, infection by Renibacterium salmoninarum progresses slowly and it may take long time before the bacterium is detectable in in infected fish. Therefore, the negative result should be interpreted with caution. Screening of wild salmonids for Renibacterium salmoninarum will continue in the coming years.
The farming intensities in PO2 and PO3 were approximately 42.4 and 53.7 tonne/km2 respectively [7]. Hardangerfjorden is located in PO3 which is one of the areas with the highest fish farming intensities in Norway. Our results indicate therefore that the prevalence of pathogen infections in postsmolt collected from this fjord was not associated with high fish farming intensity.
The current findings are in line with our previous reports that showed no apparent relationship between the prevalence of virus infection in wild salmon and the fish farming intensity or the frequency of disease outbreaks in collection areas [9-15]. These observations may indicate that wild salmon are exposed to a low infection pressure from fish farming. However, the possibility that infection may lead to rapid disappearance or altered behaviour of the infected fish, and therefore may affect the results, cannot be ruled out. Other explanation for the low prevalence of viruses in postsmolts is the time needed after virus exposure (incubation time) before the virus can be detected in tissues of fish. To verify our observations, a large PD-vaccine smolt release study was therefore conducted in 2018 and 2019. In this study, a total of 52 000 (28 000 PD-vaccinated and 24 000 control) smolt were released in rivers Etneelva and Daleelva located in production areas PO3 and PO4 respectively, which had a high number of PD cases during the release period. The survival rate of returning adult salmon in the subsequent years were determined in both vaccine and control groups and used to estimate the mortality that may be attributed to SAV infection from fish farming in release areas. The results (unpublished) from the study did not show any statistically significant differences in the mortality rate between the vaccine and the control groups and therefore support our observations that infection pressure of SAV from fish farms to wild salmon is low. Furthermore, screening smolt used in sentential cages placed in PO3 for a period of 2 weeks for ISAV and SAV infections did not reveal virus infection in these smolt.
The results in the current report showed that migrating postsmolts and sea trout had a very low occurrence of infection by 4 viruses prevalent in Norwegian aquaculture. Furthermore, Renibacterium salmoninarum was not detected in sea trout or postsmolts collected from areas with BKD-outbreaks. These findings complement and corroborate our previously reported data and may suggest that prevalence of virus infections in wild salmon at different life stages are not significantly influenced by the occurrence of these infections in fish farming. It is also suggesting that it is unlikely that wild salmon is the major reservoir that spill over these pathogens (mainly viruses) to fish farming. There are still significant gaps in our knowledge about diseases in wild fish and the interaction between farmed and wild fish [6,7]. Time series of samples of all life stages of wild salmonids from areas with different salmon farming intensities are necessary to better evaluate and understand the long-term effect of infection pressure from aquaculture on the virus prevalence in wild salmon populations. Such series will also enable us to assess changes in the prevalence due to increased fish farming activities, increased pathogen virulence, the emergence of new diseases and climate change.
6 - References
- Sommerset, I., Wiik-Nielsen, J., Moldal, T., Oliveira, V.H.S., Svendsen, J.C., Haukaas, A. & Brun, E. (2024) Norwegian Fish Health Report 2023, Norwegian Veterinary Institute Report, series #8a/2024, Norwegian Veterinary Institute.
- Hjortaas, M. J., et al. (2013). “The first detections of subtype 2-related salmonid alphavirus (SAV2) in Atlantic salmon, Salmo salar L., in Norway”. Journal of Fish Diseases, 36: 71-74.
- Rimstad, E., and Markussen, T. (2020). Infectious salmon anaemia virus-molecular biology and pathogenesis of the infection. Journal of Applied Microbiology, 129:85-97.
- Løvoll, M., et al. (2012). “Quantification of piscine reovirus (PRV) at different stages of Atlantic salmon Salmo salar production”. Diseases of Aquatic Organisms, 99: 7-U5.
- Jensen, B. B., et al. (2013). “Risk factors for cardiomyopathy syndrome (CMS) in Norwegian salmon farming”. Diseases of Aquatic Organisms, 107: 141-150.
- Taranger, G. L., et al. (2015). “Risk assessment of the environmental impact of Norwegian Atlantic salmon farming”. ICES Journal of Marine Science, 72: 997-1021.
- Grefsrud, E. S., et al. (2024). “Risk assessment of Norwegian fish farming” (In Norwegian). Rapport fra havforskningen 2024-4: Institute of Marine Research, Bergen. Available at https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-2024-4
- Nilsen, R., et al. (2017). Lakselusinfestasjon på vill laksefisk langs norskekysten i 2016; med vekt på modellbasert varsling og tilstandsbekreftelse. Rapport fra Havforskningen 1-2017: Institute of Marine Research, Bergen
- Madhun, A. S., et al. (2016). Occurrence of salmonid alphavirus (SAV) and piscine orthoreovirus (PRV) infections in wild sea trout Salmo trutta in Norway. Diseases of Aquatic Organisms, 120:109-113.
- Madhun, A. S., et al. (2018). “Prevalence of piscine orthoreovirus and salmonid alphavirus in sea-caught returning adult Atlantic salmon (Salmo salar L.) in northern Norway”. Journal of Fish Diseases, 41: 797-803.
- Madhun, A. S., et al. (2018). “Annual report on health monitoring of wild anadromous salmonids in Norway 2017; Health monitoring of returning adult salmon from river Etne, western Norway ”. Rapport fra Havforskningen 26-2018: Institute of Marine Research, Bergen. 13 pp. Available at https://www.hi.no/en/hi/nettrapporter/rapport-fra-havforskningen-en-2020-16
- Madhun, A. S., et al. (2019). Annual report on health monitoring of wild anadromous salmonids in Norway 2018; Screening of migrating Atlantic salmon (Salmo salar) postsmolts from the Trondheim fjord for viral infections. Rapport fra Havforskningen 2019-28: Institute of Marine Research, Bergen. 9 pp. Available at https://www.hi.no/en/hi/nettrapporter/rapport-fra-havforskningen-en-2019-28
- Madhun, A. S., et al. (2023). Annual report on health monitoring of wild anadromous salmonids in Norway 2022. Screening of Atlantic salmon (Salmo salar) postsmolts from Boknafjorden, Hardangerfjorden and Romsdalsfjorden for viral infections. Rapport fra Havforskningen 2023-41. Institute of Marine Research, Bergen. 10 pp. Available at https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2023-41
- Madhun, A. S., et al. (2024). "Occurrence of salmonid alphavirus and piscine orthoreovirus-1 infections in migrating salmon (Salmo salar L.) post-smolt in western Norway." Journal of Fish Diseases 47: e13874.
- Garseth, Å. H., et al. (2013). “Piscine reovirus (PRV) in wild Atlantic salmon, Salmo salar L., and sea-trout, Salmo trutta L., in Norway”. Journal of Fish Diseases, 36: 483-493.
- Garseth, Å. H., et al. (2013). “Phylogenetic evidence of long distance dispersal and transmission of piscine reovirus (PRV) between farmed and wild Atlantic salmon”. PloS One, 8: e82202.
- Garseth, Å. H., et al. (2012). “Piscine myocarditis virus (PMCV) in wild Atlantic salmon Salmo salar”. Diseases of Aquatic Organisms, 102: 157-161.