Kontroll med lakselus har lenge vært den største utfordringen for videre utvikling av en bærekraftig næring. Forebyggende tiltak er forventet å spille en økende rolle, men erfaringen tilsier at medikamentell behandling fremdeles vil være et viktig verktøy i en effektiv lusestrategi. Dagens kontrollmuligheter er begrenset av uønsket miljøeffekter eller resistensproblematikk knyttet til bruk av kjemikalier. Dette prosjektet har testet om lysaktiverte kjemikalier brukt i bl.a. human kreftmedisin kunne være en effektiv og miljøvennlig behandlingsmetode mot lakselus. Prosjektet besto av tre deler, som først undersøkte den direkte effekten av stoffer på lakselus (screeningsfase) hvoretter utvalgte stoffer ble testet på luseinfisert fisk og til slutt på organismer fra miljøet rundt merden. Ni ulike forbindelser ble testet, hvor tre av de testede forbindelsene induserte dødelighet når de ble brukt direkte på lakselus, men induserte ikke tap av lakselus når de ble brukt på infisert fisk. De testede forbindelsene hadde ingen negative effekter på fiskevelferden, induserte heller ikke dødelighet eller endret atferd hos strandreker som ble brukt som eksempel på en organismer som kanskje kunne påvirkes. Vi konkludere at bruk av fotoaktiverte forbindelser kan være mulig å bruke for å eliminere lakselus fra fisk i akvakulturmiljøer, men ytterligere studier er nødvendige.
A novel lice control method: photochemical treatment (Photolice)
Report series:
Rapport fra havforskningen 2023-31
ISSN: 1893-4536
Published: 23.06.2023
Project No.: 15775
On request by: Fiskeri- og havbruksnæringens forskningsfinansiering
Reference: 901689
Research group(s):
Smittespredning og sykdom
Subject:
Lakselus,
Laks i oppdrett
Program:
Miljøeffekter av akvakultur
Research group leader(s):
Bjørn Olav Kvamme (Smittespredning og sykdom)
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Terje Svåsand
Norsk sammendrag
Summary
Control of salmon lice has long been the biggest challenge for the further development of a sustainable industry. Preventive measures are expected to play an increasing role, but experience suggests that drug treatment will still be an important tool in an effective lice strategy. Today's control options are limited by unwanted environmental effects or resistance issues linked to the use of chemicals. This project has tested whether light-activated chemicals used in e.g. human cancer medicine could be an effective and environmentally friendly treatment method against salmon lice. The project consisted of three parts, which first examined the direct effect of substances on salmon lice (screening phase), after which selected substances were tested on lice-infected fish and finally on organisms from the environment around the cage. Nine different compounds were tested, where three of the tested compounds induced mortality when applied directly to salmon lice but did not induce loss of salmon lice when applied to infected fish. The tested compounds had no negative effects on fish welfare, nor did they induce mortality or change the behavior of rock-pool shrimps, which were used as an example of an organism that might be affected. We conclude that the use of photoactivated compounds may be possible to eliminate salmon lice from fish in aquaculture environments, but further studies are necessary.
1 - Project Information
1.1 - Background
The primary sustainability concern for salmon aquaculture remains the issue of salmon lice, and there are currently challenges with performing delousing events to control and remove lice in farms. Historically, delousing using chemotherapeutants has been common practice, however in recent years the paradigm has shifted towards alternative methods due to the rising resistance in lice populations (and therefore decreasing efficacy of active compounds), and the flow-on effect on the surrounding environment (Aaen et al. 2015, Parsons et al. 2020, Torrissen et al. 2013). Alternative methods for control most commonly used today are thermal or mechanical treatment, a combination of both, or hydrogen peroxide; however, evidence has already emerged on the severe risk on welfare and mortality caused by these approaches (Overton et al. 2018). Further worsening the issue, the mortality rates of salmon after treatment by thermal or mechanical delousing can be 5.4 and 6.3 times higher than medicinal treatment (Walde et al. 2021). This project investigated the development of a ground-breaking solution for chemical delousing in salmon aquaculture, that has the potential for high efficacy against attached lice and minimal welfare or environmental impacts, which can be incorporated into current practices for delousing using well-boats or similar approaches. The project tested the use of photochemical treatments as a means for killing salmon lice - briefly, photosensitising compounds were used for generating light-induced chemical reactions (mainly mediated by Reactive Oxygen Species (ROS)) that are toxic to organisms that contain the photosensitising compounds. Treatments are performed as a two-step process, where the compound is first introduced into the target (the louse) using bath treatments, followed by an activation step by illumination by which the toxicity is created. Photochemical treatments are used today in human medicine for the treatment of several types of cancer. There are also studies on using photochemical treatments for killing microorganisms, insect larvae and fish parasites in ponds. The broad-spectrum use of light-induced chemical reactions enables the development of this technique to target salmon lice while avoiding impacts on non-target organisms.
Photochemical compound function
A photosensitising compound (photosensitiser) is defined as a chemical entity that, upon absorption of light, induces a chemical or physical alteration of another chemical entity. Many types of chemical structures can be used as photosensitising compounds. Several synthetic photosensitisers are approved for use in human medicine (mainly based on porphyrin structures), but also many natural compounds or derivatives (e.g. chlorophyll derivatives; hypericin, curcumin) can be used as photosensitisers. Using photosensitisers based on natural compounds for treatments in fish-farming could have an advantage that the substances should be easily and rapidly broken down to naturally occurring metabolites and thus cause limited environmental concerns. Photosensitiser derivatives can easily be synthesized to influence the water/lipid solubility, amphiphilicity, pKa, and stability of the compounds. These parameters will determine e.g. the affinity of the photosensitiser molecules for various biological structures, uptake into organisms, cells, and tissues, biodistribution and pharmacokinetics.The cytotoxic effect of the photochemical treatment is usually mediated mainly through the formation of singlet oxygen (1O2). This reactive intermediate has a very short lifetime in biological systems (<0.04 μ s). Thus, the primary toxic effect of PDT is executed only during light exposure and in the very near vicinity of the photosensitiser molecules. Singlet oxygen reacts with and oxidizes proteins (histidine, tryptophan, methionine, cysteine, tyrosine), DNA (guanine), unsaturated fatty acids, and cholesterol (Fig. 1).
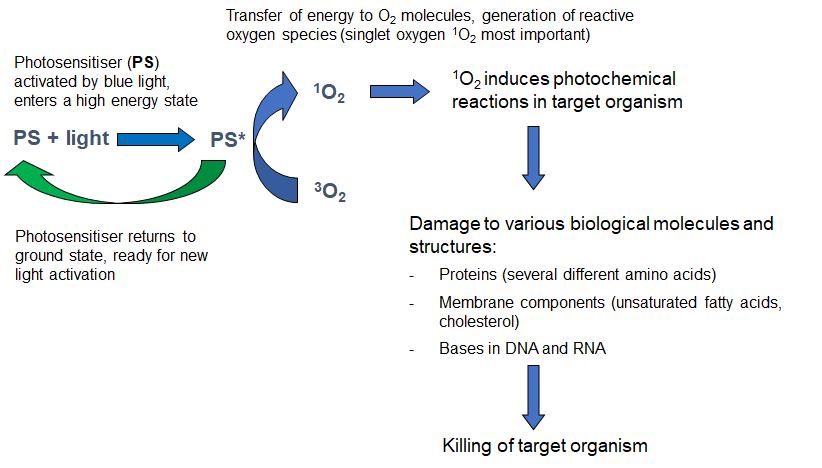
Possible applied use in salmon aquaculture against salmon lice
Depending on the photosensitising compound used, activation can be induced by light of different wavelengths. In vertebrate animal tissues the light penetration will increase with the wavelength, thus red light will penetrate deeper into the tissue (up to about 1 cm), while blue light will only penetrate around 1 mm into the tissue. Since salmon lice are relatively flat and transparent animals, illumination with blue light holds the best potential to kill the lice without harming the fish.
In contrast to what is the case in tissues, blue light penetrates well through water, so that e.g. illumination of fish within a well boat or sea cage should be possible with a number of illumination sources. In addition, one could envision using covers with reflecting materials inside the well-boat or in tubing as fish are pumped out of the boat and back to the cage to ensure even illumination through the treated water.
Photosensitisers may be designed to attach to or to be taken up non-specifically by the sea louse. Molecules that are introduced into the body cavity of lice are distributed within the animals within a short period of time (Dalvin et al. 2009) giving the possibility for systemic toxicity upon illumination. Photosensitisers may however also be designed to target egg strings of the salmon lice. To this end a possible approach could be to attach photosensitisers to polymers (preferably naturally occurring polymers) or other types of carriers making them “sticky”. Photochemical treatments also have the potential to kill free-living stages of salmon lice. In addition, it could be possible to add photosensitisers to fish feed in the same way as for certain of the conventional drugs used in treatment of salmon lice, but for most applications this does not seem optimal. Since the toxic effect of the treatment can efficiently be turned on and off by illumination, the uptake and distribution kinetics of the compounds could be used as a means for generating selectivity between the salmon lice and the fish. For example, if the photosensitiser is attached, or taken up and distributed more rapidly in the salmon lice, illumination rapidly after addition of the compound could lead to damage mainly to the salmon lice without harming the fish. It should also be noted that the proposed mechanism differs from published experiments with light induced degradation of known salmon lice chitin synthesis inhibitors (Sydnes et al. 2023).
This treatment approach is not expected to generate development of resistance in salmon lice because of the mechanisms in which the compound interacts with the louse. The photosensitisers conceived used in the present invention would not be expected to interact with specific target molecules in the salmon lice, but rather target in a more “generic” physicochemical way, based on e.g., hydrophobicity/hydrophilicity or charge. This means that there is great flexibility in designing molecules with optimal properties for selectively killing salmon lice, since the photosensitiser molecules unlike many small molecule drugs, do not need to bind to specific target molecules. It also means that it may be difficult for the salmon lice to develop resistance to the photosensitisers since e.g. mutations in one target protein would not be sufficient for generating resistance. Also, the ROS induced by illumination of the photosensitiser represent a potent and rather nonspecific toxicity mechanism that it is difficult to develop resistance against, as has also been shown in human cells when photochemical reactions are used e.g. for cancer treatment.
Safety considerations for salmon and non-target organisms
The effect of the treatment can be easily adjusted by varying the light dose (i.e. illumination time or intensity). Illumination times will depend on the type and concentration of photosensitiser used but could typically be in the range of 10 s to 2 h. Most photosensitisers are inactivated by long term or high levels of illumination (so-called photo-bleaching, analogous to being ‘burnt out’), meaning that their ability to induce toxic photochemical reactions can be inactivated shortly after, or even during, the treatment. Thus, it may be possible to adjust the concentration of photosensitiser under the treatment in such a way that the illumination dose sufficient for generating the mortality effect in salmon lice will also inactivate the photosensitiser molecules. Under such conditions one could also envision exploiting normal sunlight as the illumination source, since the total therapeutic effect achieved will, within certain limits, be more or less independent of the exact intensity of the illumination, since a lower light intensity can be compensated by a longer illumination time, and since over-treatment is avoided by photo-bleaching of the sensitiser. Photosensitisers are usually fluorescent, so it is possible to monitor the progress of the treatment by an easy in-process analysis since photo-bleaching will lead to the loss of fluorescence from the photosensitiser.
A benefit of affecting non-target organisms could be that the treatment could function against other pathogens on the surface of a salmon e.g. those present in winter sores, a substantial source of mortality in salmon production. After the treatment procedure, the extended impact on non-target animals should be low, as many of the relevant photosensitisers have very low toxicity when organisms containing the sensitisers are not exposed to light, meaning that the difference between a dose giving systemic toxicity in the fish or other non-target organisms and the dose necessary for the light induced treatment effect can be very large (often up to > 100 x).
1.2 - Project objectives
The main objective of the project was to explore the principle of using photo-activated compounds to induce lethality in salmon lice on Atlantic salmon.
Specifically, the project should:
1. Identify photo-activated compounds and validate their efficacy in salmon lice exploring a range of compounds and light treatment with respect to time and dose.
2. Test select compounds in Atlantic salmon to ensure suitability for fish welfare.
3. Test an effective photo activated compound on lice attached to fish under conditions similar to a well boat.
4. Perform an environmental risk analysis of selected compounds and procedures.
5. Determine LD50 values for non-target crustaceans.
2 - Identification of photo-activated compounds with efficacy against salmon lice (WP1).
The aim of the WP was to select compounds that lead to mortality in lice with high efficiency and characterise the properties with regards to suitable treatment of farmed fish.
2.1 - Screening using bath method.
The experiments were performed placing lice (Preadult (PA), adult male (M) and adult female (F) lice) in seawater containing of test compounds and subsequently exposed to activation of the compounds by light. The experiments were performed as a screen to test a range of doses, incubation time, aeration (addition of oxygen to treatment water is expected to enhance efficiency) and duration of light exposure as illustrated (Table 1).
Table 1. Example of experimental set-up for bath treatments.
| Bottle | compound | Exp time | Dose | Light | Animals | Temp |
|---|---|---|---|---|---|---|
| 1 | A | 10 | High | Short | 3F, 3M, 6PA | 12 C |
| 2 | A | 120 | Low | Long | ||
| 3 | A | 10 | High | Short | ||
| 4 | A | 120 | Low | Long | ||
| 5 | B | 10 | High | Short | ||
| 6 | B | 120 | Low | Long | ||
| 7 | B | 10 | High | Short | ||
| 8 | B | 120 | Low | Long | ||
| 9 | C | 10 | High | Short | ||
| 10 | C | 120 | Low | Long | ||
| 11 | C | 10 | High | Short | ||
| 12 | C | 120 | Low | Long |
Compounds were dissolved in the solvent dimethyl sulfoxide (DMSO) and diluted in seawater. Animals were kept in darkness during the exposure (Exp Time) Negative control groups (not shown in table were likewise exposed to seawater containing DMSO (max 1%) handling and light treatment (13 mW/cm2). No negative effects of DMSO at this concentration were observed. Exposure of lice was performed in small disposable plastic beakers kept at 12 °C. Aeration was achieved by placing small aeration stones in the incubation beaker and bubbling with atmospheric gas.

After light treatment (Fig. 1) lice were rinsed in seawater, and lice mortality or ability to adhere to the side of the glass was observed after 30 min, 24 h and 72 h.
In total 9 compounds were tested. These included hydrophobic compounds, symmetric hydrophilic (charged) compounds and several types of amphiphilic compounds (hydrophilic on one side of the molecule, hydrophobic on the other side). Molecules both of natural and of synthetic origin were testes. Of the 9 compounds tested three compounds (compound A, B and C, all amphiphilic) led to mortality in the highest doses tested. These compounds were tested on fish in WP2.
2.2 - Injections
Based on the initial screen which had revealed few good candidates and only when used relatively large concentrations based on findings in other animals, it was hypothesized that getting the compound into the louse could be problematic. An alternative to bath treatment was thus introduced. Adult lice male and female lice were injected using a thin glass needle. This is a well-established technique developed in to introduce double stranded RNA into lice (RNA interference experiments) but can be used to inject any compound in the hemolymph of lice (Fig. 2) from where it is spread throughout the body (Dalvin et al 2009).

Mortality associated with the method is less than 5%. After injection lice were left in sea water for x min before light exposure. A total of four compounds were tested and two types of light (blue light of 400-440 nm, 13 mW/cm2, red light of 640-660 nm, 25 mW/cm2 ) (Fig. 1 and 3). To account for possible differences in photosensitiser metabolism (and possible detoxification) in the salmon louse, both natural substances (probably easily metabolized) and fully synthetic compounds (not metabolised in mammals) were tested.
3 - Testing of selected photo-activated compounds in Atlantic salmon (WP2).
The aim of the WP was to test whether the selected compounds and treatment regime developed in WP1 would a) cause any decrease in fish health or welfare and b) efficiently remove lice from fish.
Testing of negative effects on fish was performed as the main fish experiment, except that it was performed on fewer fish which were also not infected with lice. The test also offered the opportunity to optimize practical set-up including handling of light (light was kept at a minimum during incubation) and suitable levels of oxygen (Fig.4). One of the three compounds gave rise to acute mortality in fish at the highest dose (but the same tank had problems with oxygenation so unclear what the real reason was). No other negative effects of fish health or welfare was observed during the experiment or during the 1-week monitoring period.

The main fish experiment was performed on Atlantic salmon infested with salmon lice five weeks in advance to produce adult lice. The compound that had caused problems in the previous experiment was very similar to another one tested, hence it was excluded. The main fish experiment carried out without problems, i.e. the amount of salmon lice was appropriate (approximately 12 lice per fish, 400 g fish) and incubation (1.5 h in darkness) with compounds (two, two concentrations), oxygenation and light treatment (tank lights: Phillips TL-D LIFEMAX Super 80 18W fluorescent tubes. Light intensity was 70.4 ± 2.2 µmol m-2 s-1 as measured using a LI-1500 logger equipped with an LI-193 spherical quantum underwater radiation sensor (LI-COR Biosciences, USA)) just below the waters’ surface) were carried out as planned. All the fish tolerated the treatment without problems. Unfortunately, we could not detect any delicing effect on the fish.
4 - Environmental risk analysis of selected compounds (WP3).
The aim of the WP was to assess any negative effects the novel treatment method may have on non-target organisms in the environment. Although these compounds should be used and inactivated in well boats, there will always be a risk of accidental loss and hence effects on environmental targets should be evaluated.
A LD50 study was performed on rockpool shrimp (Fig. 5) according to protocols developed at the IMR (Parsons et al 2020, Samuelsen et al 2020).

Here the effect of treatment water before, after illumination activation and post-bleach in the relevant concentrations as used in experiments with fish were compared. Bleaching was performed by exposing treatment water to bright daylight (Fig. 6).

No negative effects on shrimp behaviour, moulting or feeding were observed during the experimental period lasting 30 days after exposure.
5 - Key Findings
The project investigated the efficiency of photo-activated chemicals to remove mobile stages of salmon lice from fish in a setting applicable to Atlantic salmon kept in aquaculture. The selected compounds were tested for efficacy against lice using bath methods and for some compounds also injections.
- A few of the tested compounds did induce mortality when applied directly to salmon lice but did not induce loss of salmon lice when applied to infested fish
- The tested compounds did not have negative effects on fish welfare, neither did they induce mortality, or changed behaviour in non-target organisms (rock pool shrimp)
- Three compounds were injected into the salmon lice, but this approach elicited no toxicity upon illumination of the injected salmon lice.
- The tested compounds are expected to lead to disruption of basic cellular mechanisms; hence it is unknown why only minor effects were observed in salmon louse. This may be due to physiology and metabolism of lice or delivery of the compounds into cells.
- The use of photoactivated compounds may be feasible to apply to eliminate salmon lice from fish in an aquaculture setting but further studies and development of delivery are needed.
6 - References
Aaen, S.M., Helgesen, K.O., Bakke, M.J., Kaur, K., Horsberg, T.E. (2015) Drug resistance in sea lice: a threat to salmonid aquaculture. Trends in Parasitology. 31, 72-81.
Barrett, L.T., Bui, S., Oppedal, F., Bardal, T., Olsen, R.E., Dempster, T. (2020) Ultraviolet-C light suppresses reproduction of sea lice but has adverse effects on host salmon. Aquaculture. 520, 734954.
Dalvin, S., Frost, P., Biering E., Hamre, L. A., Eichner C., Krossøy, B., Nilsen, F. (2009) Functional characterisation of the maternal yolk-associated protein (LsYAP) utilising systemic RNA interference in the salmon louse (Lepeophtheirus salmonis) (Crustacea: Copepoda). International journal for parasitology. 69 1407-1415.
Overton, K., Dempster, T., Oppedal, F., Kristiansen, T.S., Gismervik, K., Stien, L.H. (2018) Salmon lice treatments and salmon mortality in Norwegian aquaculture: a review. Reviews in Aquaculture. 11, 1398-1417.
Parsons, A.E., Escobar-Lux, R.H., Sævik, P.N., Samuelsen, O.B., Agnalt, A.L. (2020) The impact of anti-sea lice pesticides, azamethiphos and deltamethrin, on European lobster (Homarus gammarus) larvae in the Norwegian marine environment. Environmental Pollution. 264, 114725.
Samuelsen, O. B., Parsons A. E., Agnalt A., Tjensvoll, T., Lunestad, B.T., Hannisdal, R. Mortality in the rockpool shrimp Palaemon elegans following long-term exposure to low doses of the anti-parasitic drug teflubenzuron. Aquaculture Environment Interactions. 12, 2020.
Sydnes, M. O., Eikemo V., Espedal, P. G., Sydnes, L. K., Nilsen F. (2023) Evaluation of photodegradable chitin synthetase inhibitors for the treatment of salmon lice (Lepeophtheirus salmonis). Aquaculture. 569, 739372
Torrissen, O., Jones, S., Asche, F., Guttormsen, A., Skilbrei, O.T., Nilsen, F., Horsberg, T.E., Jackson, D. (2013) Salmon lice – impact on wild salmonids and salmon aquaculture. Journal of Fish Diseases. 36, 171-194.
Walde, C. S., Jensen, B.B., Pettersen, J.M., Stormoen, M. (2021) Estimating cage‐level mortality distributions following different delousing treatments of Atlantic salmon (salmo salar) in Norway. Journal of Fish Diseases. doi: 10.1111/jfd.13348